Abstract
Purpose:
To evaluate the clinical features and risk factors of hemorrhagic complications in polypoidal choroidal vasculopathy (PCV) using spectral domain-optical coherence tomography (SD-OCT) and indocyanine green angiography (ICGA).
Methods:
We respectively reviewed the data from 43 patients (45 eyes) diagnosed with PCV who received ICGA between January 2010 and October 2013. The patients were divided into 2 groups: 16 patients (17 eyes) with subretinal hemorrhage (su-bretinal hemorrhagic PCV group) and 27 patients (28 eyes) without subretinal hemorrhage (control group). Based on the ICGA and SD-OCT findings, the number, morphology, location, size of polyps, pigment epithelial detachment (PED), and serous reti-nal detachment (SRD) were measured and compared between the 2 groups. We also analyzed systemic diseases and history of antithrombotic agents associated with subretinal hemorrhage in PCV.
Results:
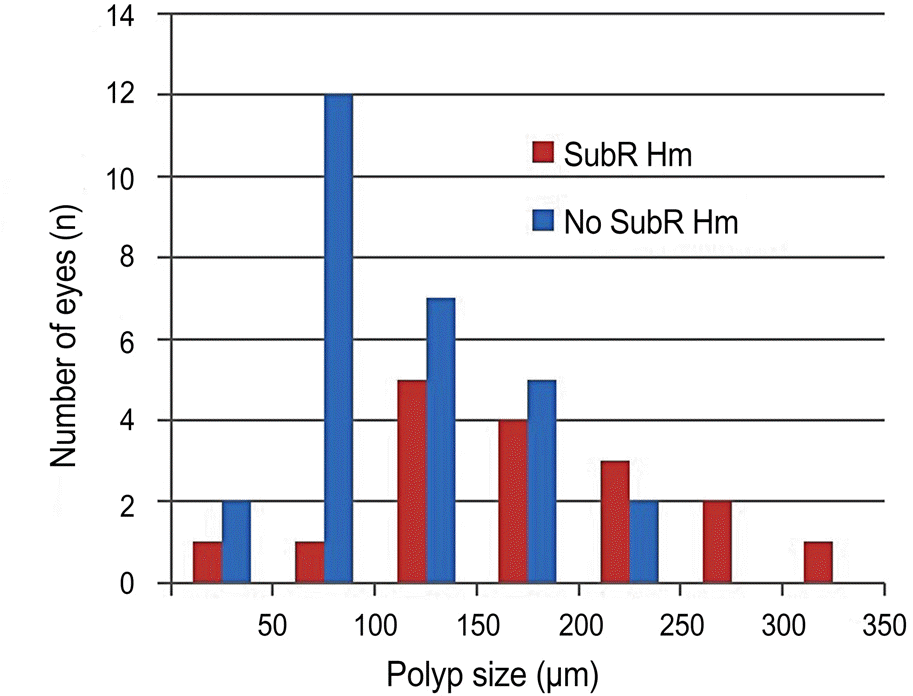
The size of polyps measured by ICGA was significantly different between the 2 groups ( p = 0.006). As the size of polyps increased, the size of subretinal hemorrhage, height of PED, base diameter and height of SRD increased ( p < 0.05). No stat-istical correlation with systemic diseases and antithrombotic agents was observed ( p > 0.05).
Conclusions:
The patients in the subretinal hemorrhagic PCV group had larger-sized polyps than the patients in the control group. This result suggests that eyes with larger-sized polyps are at risk for hemorrhagic complications and require more careful follow-up and observation in PCV treatment-naïve patients.
Go to : 
References
1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic poly-poidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.

2. Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997; 115:478–85.

3. Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degenera-tion. Arch Ophthalmol. 1999; 117:1503–10.

4. Tateiwa H, Kuroiwa S, Gaun S, et al. Polypoidal choroidal vascul-opathy with large vascular network. Graefes Arch Clin Exp Ophthalmol. 2002; 240:354–61.

5. Woon WH, Fitzke FW, Bird AC, Marshall J. Confocal imaging of the fundus using a scanning laser ophthalmoscope. Br J Ophthalmol. 1992; 76:470–4.

6. Bartch DU, Weinreb RN, Zinser G, Freeman WR. Confocal scan-ning infrared laser ophthalmoscopy for indocyanine green angiography. Am J Ophthalmol. 1995; 120:642–51.
7. Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green vid-eoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15:100–10.

8. Sa HS, Cho HY, Kang SW. Optical coherence tomography of idio-pathic polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2005; 19:275–80.

9. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal cho-roidal vasculopathy. Br J Ophthalmol. 2005; 89:602–7.

10. Imamura Y, Engelbert M, Iida T, et al. Polypoidal choroidal vascul-opathy: a review. Surv Ophthalmol. 2010; 55:501–15.

11. Kokame GT. Polypoidal choroidal vasculopathy-a type I poly-poidal subretinal neovasculopathy. Open Ophthalmol J. 2013; 7:82–4.
12. Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vas-culopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010; 29:19–29.
13. Byeon SH, Lee SC, Oh HS, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008; 52:57–62.

14. Koh AH. Expert PCV Panel. Chen LJ, et al. Polypoidal choroidal asculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013; 33:686–716.
15. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculop-athy: natural history. Am J Ophthalmol. 2002; 133:639–48.
16. Jung JH, Lee JK, Lee JE, Oum BS. Results of vitrectomy for break-through vitreous hemorrhage associated with age-related macular degeneration and polypoidal choroidal vasculopathy. Retina. 2010; 30:865–73.

17. Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982; 94:762–73.

18. Cho HJ, Lee DW, Cho SW, et al. Hemorrhagic complications after intravitreal ranibizumab injection for polypoidal choroidal vasculopathy. Can J Ophthalmol. 2012; 47:170–5.

19. Hirami Y, Tsujikawa A, Otani A, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007; 27:335–41.

20. Lee PY, Lee WK. Changes of network vessels after photodynamic therapy in polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2006; 47:1751–8.
21. Lee JK, Lee JW, Lee JE, Oum BS. Intravitreal bevacizumab with or without photodynamic therapy for the treatment of polypoidal cho-roidal vasculopathy. J Korean Ophthalmol Soc. 2010; 51:684–92.

22. Cackett P, Wong D, Yeo I. A classification system for polypoidal choroidal vasculopathy. Retina. 2009; 29:187–91.

23. Lee JW, Kim IT. Epidemiologic and clinical characteristics of pol-ypoidal choroidal vasculopathy in Korean patients. J Korean Ophthalmol Soc. 2007; 48:63–74.
24. Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49:4729–37.

Go to : 
 | Figure 1.60 year old man (A-C) and 84 years old man (D-F) with massive subretinal hemorrhage in a polypoidal choroidal vasculopathy. (A, D) Color fundus photograph showing orange-red polypoidal region with massive subretinal hemorrhage. (B, E) Indocyanine green angiography showing a polypoidal macular consistent with the orange-red nodules seen on fundus photography. (C, F) Spectral domain-optical coherence tomography showing a dome like elevation of highly reflective band corresponding to the detached retina pigment epithelium. |
 | Figure 2.(A) Indocyanine green angiography findings showing dense clusters of numerous, small, hyperfluorescent dots resembling microaneurysmal dilatations (arrow). (B) Relatively large aneurismal dilations (arrow). (C) Atypical vessel deformations, loop like configurations or dilations (arrow) in polypoidal choroidal vasculopathy. |
Table 1.
Demographics and clinical information of the study group
| Parameter | Group (%) | Total (%) | p-value | |
|---|---|---|---|---|
| Subretinal hemorrhagic PCV | Non-subretinal hemorrhagic PCV | |||
| Number of patients | 16 | 27 | 43 | |
| Number of eyes | 17 | 28 | 45 | |
| Mean age (years) | 69.7 ± 2.1 | 62.1 ± 3.6 | 64.9 ± 2.4 | |
| Gender (%) | ||||
| Male | 12 (75) | 19 (70.4) | 31 (72.1) | |
| Female | 4 (25) | 8 (29.6) | 12 (27.9) | |
| Right:left (eyes) | 5:12 | 17:11 | 22:27 | |
| BCVA (log MAR) | 0.85 ± 0.15 | 0.59 ± 0.09 | 0.69 ± 0.08 | 0.127† |
| Underlying disease (%) | ||||
| Hypertension | 9 (56.3) | 11 (40.7) | 21 (48.8) | 0.361∗ |
| DM | 3 (18.8) | 5 (18.5) | 8 (18.6) | 1.000∗ |
| Heart attack/CVA | 4 (25.0) | 3 (11.1) | 7 (16.2) | 0.394∗ |
| Cancer | 2 (12.5) | 1 (3.7) | 3 (6.9) | 0.545∗ |
| Anticoagulant | 4 (25.0) | 2 (7.4) | 6 (13.9) | 0.174∗ |
Table 2.
Comparison of ICGA findings between subretinal hemorrhage group and control group
| Subretinal hemorrhagic PCV (n = 17) | Non-subretinal hemorrhagic PCV (n = 28) | Total (n = 45) | p-value | |
|---|---|---|---|---|
| Polyps location | 0.535∗ | |||
| Subfovea | 8 (47.1) | 11 (39.3) | 19 (42.2) | |
| Juxtafovea | 3 (17.6) | 10 (35.7) | 13 (28.9) | |
| Extrafovea | 6 (35.3) | 7 (25.0) | 13 (28.9) | |
| Number of polyps | 0.414∗ | |||
| n ≤ 2 | 7 (41.2) | 8 (28.6) | 15 (33.3) | |
| 3 ≤ n < 5 | 2 (11.8) | 8 (28.6) | 10 (22.2) | |
| n ≥ 5 | 8 (47.1) | 12 (42.9) | 20 (44.4) | |
| Polyps morphology | 0.744∗ | |||
| Cluster, microaneurysm | 8 (47.1) | 17 (60.7) | 25 (55.6) | |
| Dilated, macroaneurysm | 7 (41.2) | 8 (28.6) | 15 (33.3) | |
| Vessel malformation | 2 (11.8) | 3 (10.7) | 5 (11.1) | |
| Polyps size (μ m) | 178 ± 16 | 121 ± 9 | 142 ± 9 | 0.006† |
| ≤150 | 7 (41.2) | 21 (75) | 28 (62.2) | |
| 151-250 | 7 (41.2) | 7 (25) | 14 (31.1) | |
| >250 | 3 (17.6) | 0 (0) | 3 (6.7) | |
Table 3.
Comparison of SD-OCT findings between subretinal hemorrhage group and control group
| Subretinal hemorrhagic PCV | Non-subretinal hemorrhagic PCV | Total | p-value∗ | |
|---|---|---|---|---|
| PED base (μ m) | 2,815 ± 424 | 1,164 ± 137 | 1,765 ± 212 | p < 0.001 |
| PED height (μ m) | 657 ± 93 | 171 ± 17 | 347 ± 50 | p < 0.001 |
| SRD base (μ m) | 3,762 ± 571 | 1,940 ± 272 | 2,630 ± 298 | 0.004 |
| SRD height (μ m) | 437 ± 78 | 118 ± 19 | 234 ± 38 | p < 0.001 |
Table 4.
Relationship between the size of polyps, subretinal hemorrhage, PED and SRD measured by ICGA and SD-OCT
| Polyps size (μ m) | ||
|---|---|---|
| r | p-value∗ | |
| Subretinal hemorrhage size | 0.616 | 0.008 |
| PED base | 0.202 | 0.189 |
| PED height | 0.399 | 0.007 |
| SRD base | 0.404 | 0.007 |
| SRD height | 0.466 | 0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download