Abstract
Purpose
To investigate the incidence, clinical manifestations, and risk factors of ocular graft-versus-host disease (GVHD) as well as the survival of the patients after allogeneic hematopoietic stem cell transplantation (HSCT).
Methods
The medical records of 99 patients who visited our clinic and were screened for ocular GVHD after allogeneic HSCT were reviewed retrospectively. Subjects were divided into 2 groups depending on the occurrence of ocular GVHD on slit-lamp biomicroscopy. We compared clinical manifestations and survival between the 2 groups and analyzed the risk factors associated with the development of ocular GVHD.
Results
Ocular GVHD was diagnosed in 38 patients (38.38%) at a mean of 315 days after HSCT. Out of the 38 patients who developed ocular GVHD, 22 patients (57.89%) were diagnosed with dry eye only and 16 patients (42.11%) were diagnosed with conjunctival disease. The presence of extraocular GVHD (hazard ratio (HR) 35.76, p < 0.001), the number of extraocular GVHD (HR 3.07, p < 0.001), skin GVHD (HR 2.31, p = 0.029), oral GVHD (HR 8.16, p < 0.001), and gastrointestinal tract GVHD (HR 5.00, p = 0.002) were independent risk factors of ocular GVHD. Comparisons of the survival demonstrated decreased survival of patients with conjunctival disease compared to patients without ocular GVHD and patients with dry eye only, but there was no statistically significant differences (log rank test, p = 0.208).
Conclusions
Ocular GVHD is common after allogeneic HSCl. Ihe majority of ocular GVHD occurs in the chronic stage and is associated with decreased survival. Therefore, more intensive and long-term follow-up with ophthalmic and systemic monitoring is necessary, especially in patients who have extraocular GVHD, for early recognition and proper treatment of ocular GVHD.
References
1. Lennard AL, Jackson GH. Stem cell transplantation. BMJ. 2000; 321:433–7.
2. Passweg JR, Baldomero H, Bregni M, et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant. 2013; 48:1161–7.

3. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11:945–56.
5. Ogawa Y, Okamoto S, Wakui M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999; 83:1125–30.

6. Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003; 22:S19–27.

7. Westeneng AC, Hettinga Y, Lokhorst H, et al. Ocular graft-versus-host disease after allogeneic stem cell transplantation. Cornea. 2010; 29:758–63.

8. Tabbara KF, Al-Ghamdi A, Al-Mohareb F, et al. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology. 2009; 116:1624–9.

10. Bray LC, Carey PJ, Proctor SJ, et al. Ocular complications of bone marrow transplantation. Br J Ophthalmol. 1991; 75:611–4.

11. Anderson NG, Regillo C. Ocular manifestations of graft versus host disease. Curr Opin Ophthalmol. 2004; 15:503–7.

12. Hessen M, Akpek EK. Ocular graft-versus-host disease. Curr Opin Allergy Clin Immunol. 2012; 12:540–7.

13. Franklin RM, Kenyon KR, Tutschka PJ, et al. Ocular manifestations of graft-vs-host disease. Ophthalmology. 1983; 90:4–13.

14. Mencucci R, Rossi Ferrini C, Bosi A, et al. Ophthalmological aspects in allogenic bone marrow transplantation: Sjögren-like syndrome in graft-versus-host disease. Eur J Ophthalmol. 1997; 7:13–8.

15. Kamoi M, Ogawa Y, Uchino M, et al. Donor-recipient gender difference affects severity of dry eye after hematopoietic stem cell transplantation. Eye (Lond). 2011; 25:860–5.

16. Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005; 105:2973–8.

17. Ochs LA, Miller WJ, Filipovich AH, et al. Predictive factors for chronic graft-versus-host disease after histocompatible sibling donor bone marrow transplantation. Bone Marrow Transplant. 1994; 13:455–60.
18. Remberger M, Kumlien G, Aschan J, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002; 8:674–82.

19. Kondo M, Kojima S, Horibe K, et al. Risk factors for chronic graft-versus-host disease after allogeneic stem cell transplantation in children. Bone Marrow Transplant. 2001; 27:727–30.

20. Jacobs R, Tran U, Chen H, et al. Prevalence and risk factors associated with development of ocular GVHD defined by NIH consensus criteria. Bone Marrow Transplant. 2012; 47:1470–3.

21. Pak KH, Myong YW, Rhee SW. A clinical evaluation of ocular manifestation in bone marrow transplanted patients. J Korean Ophthalmol Soc. 1991; 32:294–9.
22. Jabs DA, Wingard J, Green WR, et al. The eye in bone marrow transplantation- III- Conjunctival graft-vs-host disease. Arch Ophthalmol. 1989; 107:1343–8.
23. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006; 25:900–7.
24. Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003; 102:756–62.

25. Levine JE, Uberti JP, Ayash L, et al. Lowered-intensity preparative regimen for allogeneic stem cell transplantation delays acute graft-versus-host disease but does not improve outcome for advanced hematologic malignancy. Biol Blood Marrow Transplant. 2003; 9:189–97.

26. Hirst LW, Jabs DA, Tutschka PJ, et al. The eye in bone marrow transplantation- I- Clinical study. Arch Ophthalmol. 1983; 101:580–4.
27. Janin A, Facon T, Castier P, et al. Pseudomembranous con- junctivitis following bone marrow transplantation: immunopatho- logical and ultrastructural study of one case. Hum Pathol. 1996; 27:307–9.
Figure 1.
Cumulative incidence of ocular GVHD after allogeneic hematopoietic stem cell transplantation. Probability of developing ocular GVHD was 48.33% at 2 years. GVHD = graft-versus-host disease; HSCT = hematopoietic stem cell transplantation.

Figure 2.
Categorization of subjects who developed ocular graft-versus-host disease after allogeneic hematopoietic stem cell transplantation by clinical courses. GVHD = graft-versus-host disease.
Figure 3.
Kaplan-Meier survival curves of 3 groups according to the manifestation of ocular GVHD in patients who underwent allogeneic hematopoietic stem cell transplantation. Survival rates of groups of patients without ocular GVHD, with dry eye only, and with conjunctival disease are 79.8%, 95.5%, and 72.7% at 1 year, 56.9%, 70.9%, and 46.5% at 2 years, and 52.2%, 60.8%, and 34.9% at 3 years after allogeneic hematopoietic stem cell transplantation, respectively (log rank test, p = 0.208). GVHD = graft-versus-host disease; HSCT = hematopoietic stem cell transplantation.
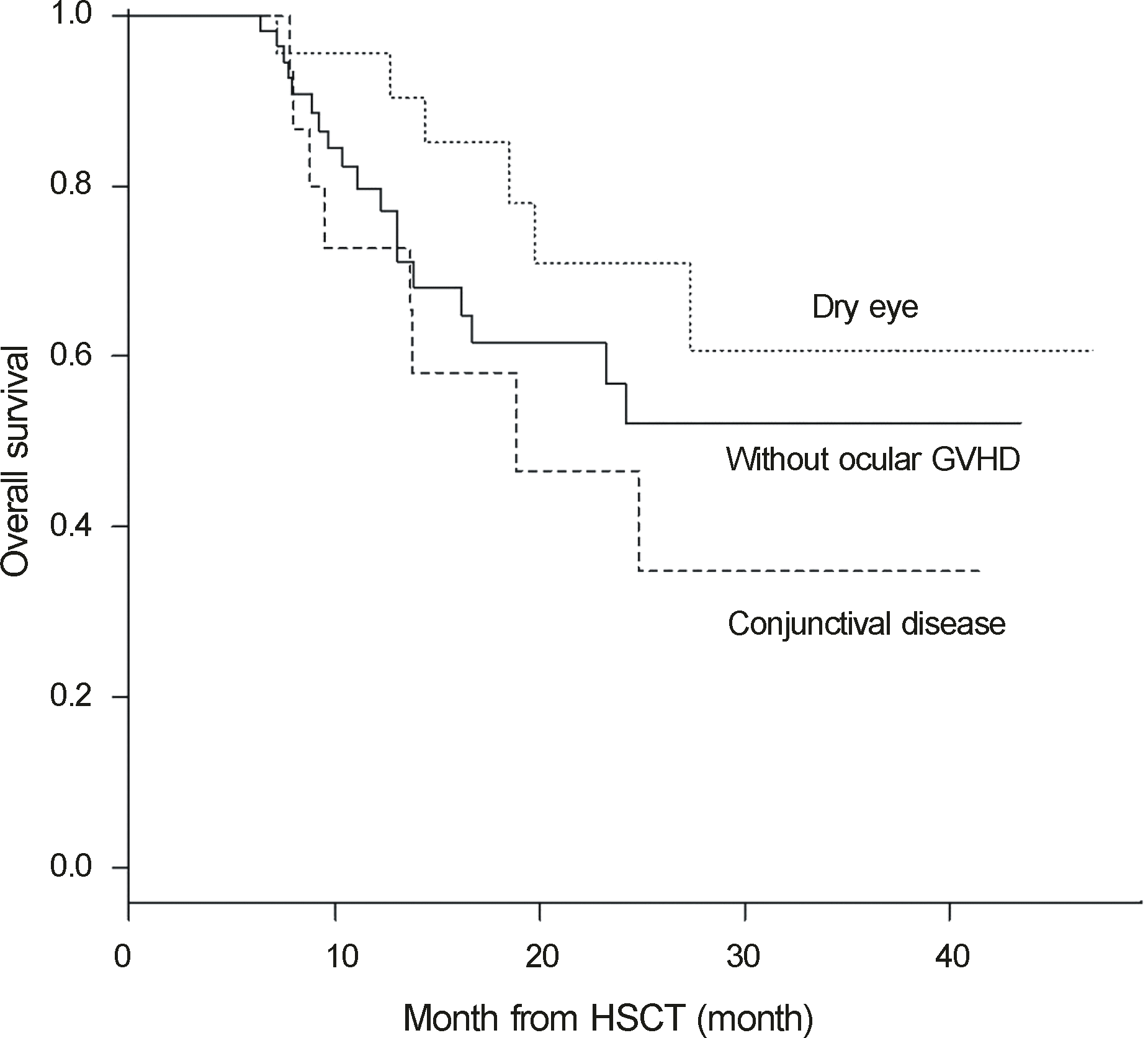
Table 1.
Characteristics of 99 subjects who underwent allogeneic hematopoietic stem cell transplantation
| Without ocular GVHD (n = 61) | With ocular GVHD (n = 38) | p-value | |
|---|---|---|---|
| Sex (n) (M/F, % of male) | 30/31 (49.18) | 26/12 (68.42) | 0.060‡ |
| Age (years) (range) | 41.40 ± 12.83, 18-64 | 41.42 ± 13.36, 18-65 | 0.905§ |
| Diagnosis* (n, %) | |||
| Myeloid hematologic malignancy | 40 (65.57) | 33 (86.85) | 0.030Π |
| Lymphoid hematologic malignancy | 17 (27.87) | 3 (7.89) | |
| Others | 4 (6.56) | 2 (5.26) | |
| Conditioning regimens (n, %) | 0.144‡ | ||
| With TBI† | 30 (49.18) | 13 (34.21) | |
| Without TBI | 31 (50.82) | 25 (65.79) | |
| Extraocular GVHD (n, %) | 32 (52.46) | 38 (100.00) | <0.001‡ |
| Onset of extraocular GVHD | 17/15 (53.13) | 12/26 (31.58) | 0.068‡ |
| (acute/chronic, % of acute onset) | |||
| Number of involved organ (%) | 0.016‡ | ||
| 1 | 14 (43.75) | 5 (13.16) | |
| 2 | 10 (31.25) | 18 (47.37) | |
| ≥3 | 8 (25.00) | 15 (39.47) | |
| Site of involved organ (n, %) | |||
| Skin | 19 (59.38) | 26 (68.42) | 0.431‡ |
| Mouth | 12 (37.50) | 24 (63.16) | 0.032‡ |
| GI tract | 6 (18.75) | 8 (21.05) | 0.810‡ |
| Liver | 14 (43.75) | 23 (60.53) | 0.161‡ |
| Others | 11 (34.38) | 10 (26.32) | 0.464‡ |
Table 2.
Risk factors of ocular graft-versus-host disease after allogeneic hematopoietic stem cell transplantation
| Risk factors |
Hazard ratio (95% confidence interval), p-value* |
||
|---|---|---|---|
| Ocular GVHD | Dry eye only | Conjunctival disease | |
| Female gender | 0.40 (0.18-0.87) | 0.26 (0.09-0.80) | 0.67 (0.22-2.03) |
| 0.021 | 0.019 | 0.474 | |
| Age | 1.00 (0.98-1.03) | 1.01 (0.98-1.05) | 1.00 (0.96-1.04) |
| 0.667 | 0.505 | 0.996 | |
| Conditioning regimens with TBI | 0.29 (0.13-0.65) | 0.34 (0.12-0.97) | 0.22 (0.06-0.81) |
| 0.002 | 0.044 | 0.023 | |
| Site of involved organ with extraocular GVHD | |||
| Skin | 2.31 (1.09-4.89) | 2.58 (0.96-6.91) | 2.21 (0.69-7.14) |
| 0.029 | 0.060 | 0.184 | |
| Mouth | 8.16 (3.20-20.82) | 11.64 (3.10-43.74) | 4.93 (1.35-18.07) |
| <0.001 | <0.001 | 0.016 | |
| GI tract | 5.00 (1.79-13.92) | 3.43 (0.64-18.48) | 7.35 (1.94-27.79) |
| 0.002 | 0.151 | 0.003 | |
| Liver | 1.58 (0.66-3.77) | 1.30 (0.40-4.20) | 1.84 (0.51-6.70) |
| 0.304 | 0.663 | 0.356 | |
| Others | 0.90 (0.40-2.02) | 0.88 (0.30-2.60) | 0.90 (0.26-3.09) |
| 0.799 | 0.820 | 0.861 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download