Abstract
Case summary
A 70-year-old man presented to our hospital with complaints of ocular pain and deterioration of the visual acuity in his left eye after injury caused by a wood branch one week previously. Visual acuity in the left eye was 20/400 at the time of the first visit. Slit lamp examination showed a central 0.7 × 2.5-mm-sized epithelial defect surrounded by cellular infiltration in the stroma. Scraping of the corneal lesion for microbiological examinations was performed. Initial Gram stain, potassium hydroxide (KOH) mount, and culture were negative. However, fungal hyphae were observed on a KOH mount of the repeated corneal scraping specimen, and a Beauveria species was suspected based on the culture. Beauveria bassiana was confirmed using a MicroSEQ® D2 large-subunit ribosomal DNA fungal sequencing kit. Natamycin eye solution was initially instilled bihourly (every two hours), but the persistent epithelial defect and progressive stromal melting finally resulted in a descemetocele. Temporary and permanent amniotic membrane transplantations were performed, and amphotericin B eye solution was administered bi-hourly (every two hours). The ulcerous lesion gradually improved with no evidence of recurrence.
References
1. Wang Q, Xu L. Beauvericin, a bioactive compound produced by fungi: a short review. Molecules. 2012; 17:2367–77.

2. Wagner BL, Lewis LC. Colonization of corn, Zea mays, by the en-tomopathogenic fungus Beauveria bassiana. Appl Environ Microbiol. 2000; 66:3468–73.
3. Ishibashi Y, Matsumoto Y, Takei K. The effects of intravenous mi-conazole on fungal keratitis. Am J Ophthalmol. 1984; 98:433–7.

4. Sachs SW, Baum J, Mies C. Beauveria bassiana keratitis. Br J Ophthalmol. 1985; 69:548–50.
5. Low CD, Badenoch PR, Coster DJ. Beauveria bassiana keratitis cured by deep lamellar dissection. Cornea. 1997; 16:698–9.

6. Kisla TA, Cu-Unjieng A, Sigler L, Sugar J. Medical management of Beauveria bassiana keratitis. Cornea. 2000; 19:405–6.

7. Tu EY, Park AJ. Recalcitrant Beauveria bassiana keratitis: confocal microscopy findings and treatment with posaconazole (Noxafil). Cornea. 2007; 26:1008–10.
8. Pariseau B, Nehls S, Ogawa GS, et al. Beauveria keratitis and bio-pesticides: case histories and a random amplification of poly-morphic DNA comparison. Cornea. 2010; 29:152–8.

9. Figueira L, Pinheiro D, Moreira R, et al. Beauveria bassiana kerati-tis in bullous keratopathy: antifungal sensitivity testing and mana-gement. Eur J Ophthalmol. 2012; 22:814–8.

10. Oh JY, Lee MJ, Wee WR, Heo JW. A case of necrotizing scleroker-atitis and endophthalmitis caused by Beauveria bassiana. Jpn J Ophthalmol. 2009; 53:551–3.

11. Jun KR, Jang MS, Park SJ, et al. A case of Beauveria bassiana keratitis. Korean J Clin Microbiol. 2007; 10:73–6.
12. Hall L, Wohlfiel S, Roberts GD. Experience with the MicroSeq D2 large-subunit ribosomal DNA sequencing kit for identification of filamentous fungi encountered in the clinical laboratory. J Clin Microbiol. 2004; 42:622–6.

13. Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diag-nosis and management. Clin Microbiol Infect. 2013; 19:210–20.

14. Ferrer C, Alió JL. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. J Ophthalmic Inflamm Infect. 2011; 1:15–22.

15. Kim SJ, Lee SB. Analysis on elderly inpatients with infectious ker-atitis: causative organisms, clinical aspects, and risk factors. J Korean Ophthalmol Soc. 2010; 51:1554–67.

16. Valencia JW, Gaitán Bustamante AL, Jiménez AV, Grossi-de-Sá MF. Cytotoxic activity of fungal metabolites from the pathogenic fungus Beauveria bassiana: an intraspecific evaluation of beauver-icin production. Curr Microbiol. 2011; 63:306–12.

17. Guarro J, Gené J. Acrophialophora fusispora misidentified as Scedosporium prolificans. J Clin Microbiol. 2002; 40:3544. author reply 3545.
Figure 1.
Initial photographs of patient's cornea. (A) On slit lamp examination, vertical linear epithelial defect surrounded by sub-epithelial and stromal infiltration with immune ring is seen at the nasal paracentral area (arrow heads). (B) Fluorescein stain reveals 0.7 × 2.5-mm- sized linear epithelial defect (arrows).
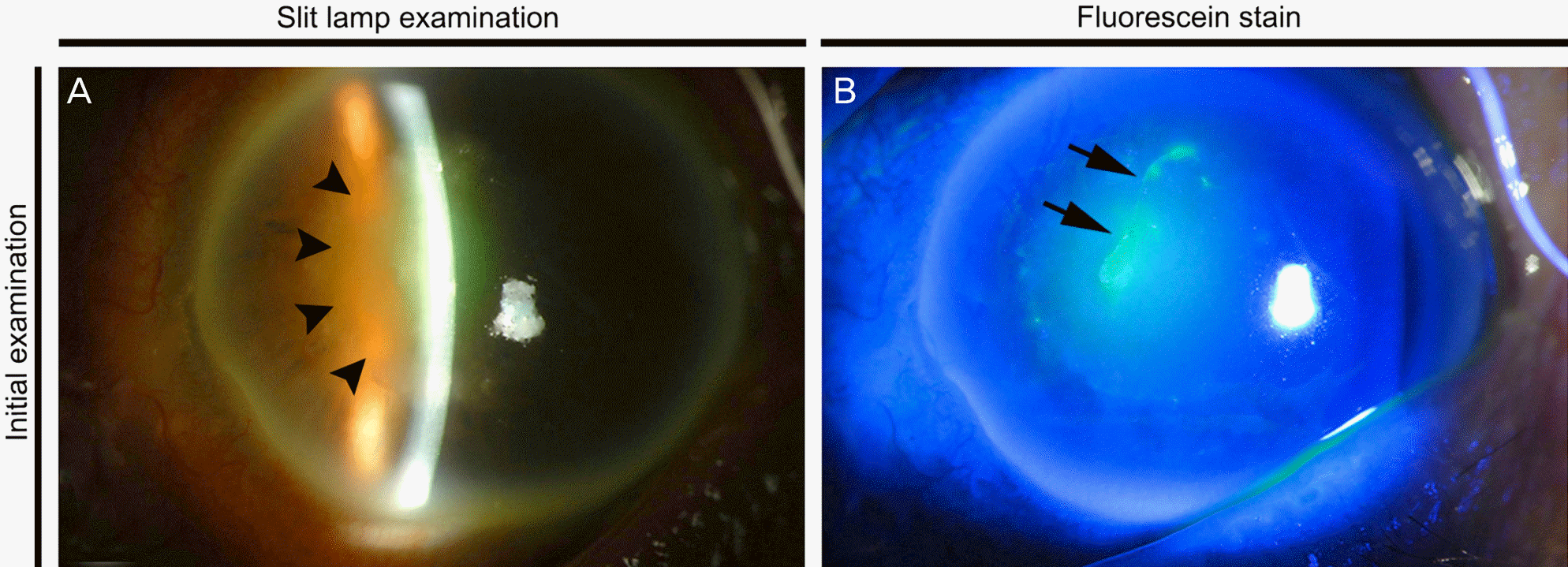
Figure 2.
(A) Direct inoculation of corneal scrapping speci-men shows white to cream color and powdery surface after 8 days of incubation at 25 °C on Sabouraud dextrose agar. (B) Microscopic findings of B. bassiana (latophenol cotton blue stain, × 1000). Narrow, delicate and septated hyphae and nu-merous microconidia are observed. The flask-shaped con-idiophores with a narrow zigzag terminal extension bearing asingle conidium at each bent point.
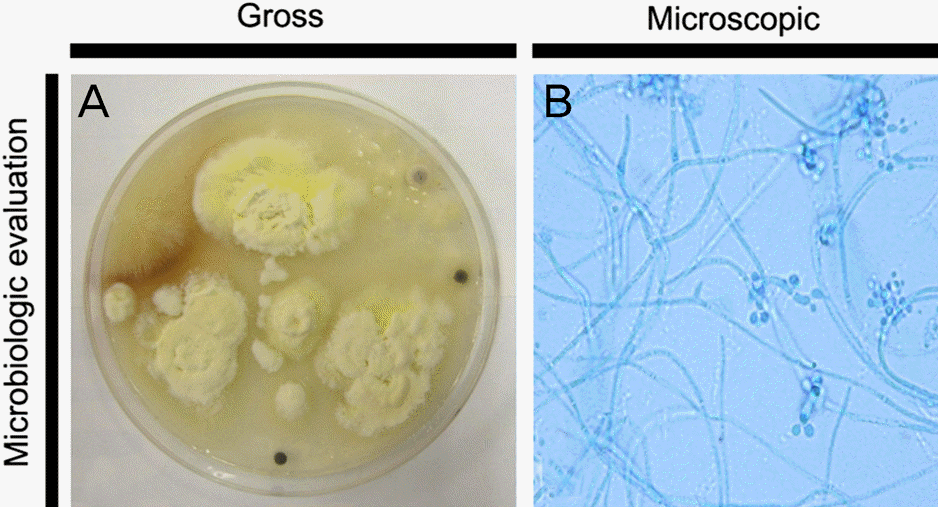
Figure 3.
Schematic diagram of fungal gene sequencing procedures. Initially, genomic deoxyribonucleic acid (DNA) was isolated form single colony, and then polymerase chain reaction (PCR) was performed for amplifying the D2 region of fungal DNA. Cycle sequencing and purifying the extension products were per-formed and sequencing of the extension products after gel elec-trophoresis were also performed. Finally, sequencing data was compared with kit libraries for identifying specific pathogen.
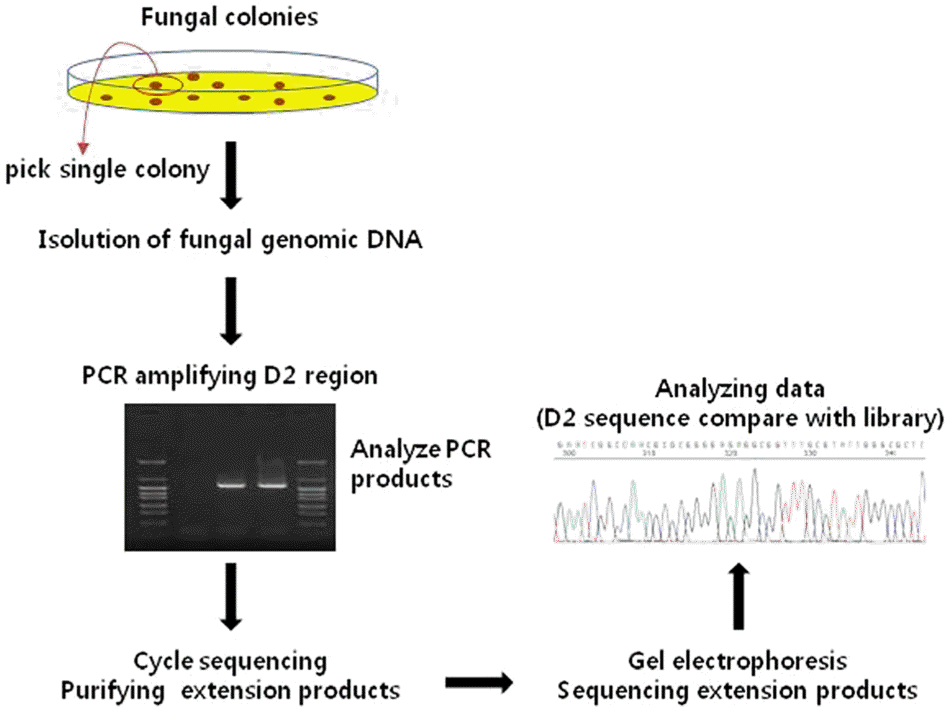
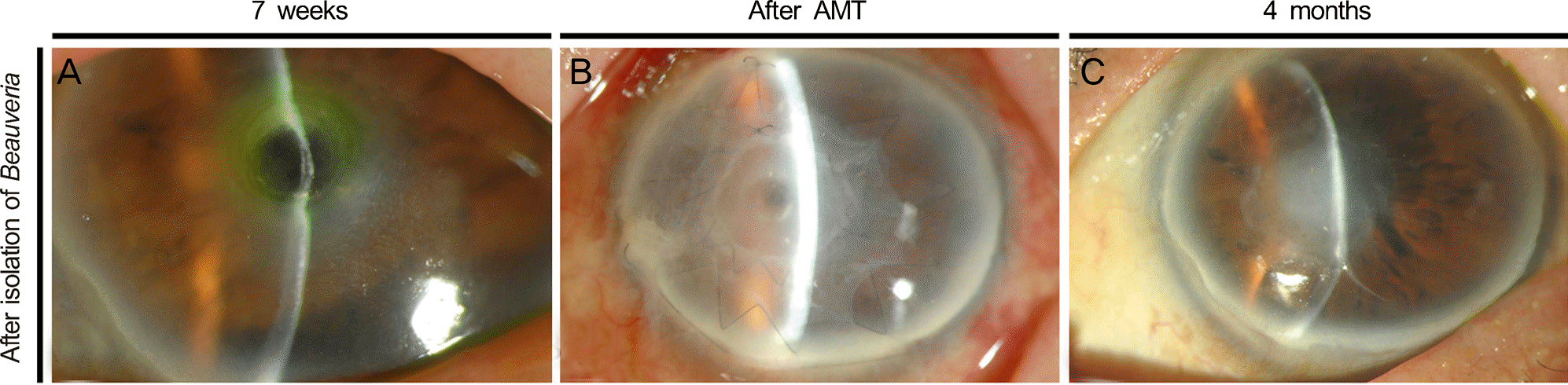
Figure 4.
(A) At 7 weeks after treatment, descemetocele occurred by progressive stromal melting. (B) Descematocele was covered after performing permanent and temporary amniotic membrane transplantation. (C) At 4 months after treatment, epithelial defect healed completely without recurrence of fungal infection. AMT = amniotic membrane transplantation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download