Abstract
Purpose
Recently, the introduction of spectral-domain optical coherence tomography (SD-OCT) has enabled measurement of retinal thickness in the posterior pole in 64 sectors. SD-OCT was used to evaluate the diagnostic effectiveness in detecting glau-comatous abnormality of visual field sensitivity. A normal value for retinal thickness was determined and then compared in corre-sponding local sectors.
Methods
Thirty healthy controls and 30 glaucoma subjects were evaluated. Macular thickness values from the 4 adjacent square cells in an 8 × 8 posterior pole retinal thickness map were averaged for a mean retinal thickness (MRT) value. A normative database was prepared using the data from the healthy eyes of this study to determine the diagnostic criteria for MRT. If the MRT value was <5% (Criteria A) or <1% (Criteria B) of the normative database, it was considered to be abnormal. The abnormalities of the MRT value for each diagnostic criteria were compared with the visual field sensitivity results in the corresponding positions.
Results
The concordance of abnormalities between MRT and visual field sensitivity at 16 measured points was low in both criteria A (Kappa value; −0.418∼0.429) and B (Kappa value; −0.363∼0.444). Based on the results of the visual field at each focal point, the sensitivities and specificities of MRT values using the 2 criteria ranged from 0% to 100%.
Go to : 
References
1. Quigley HA, Katz J, Derick RJ, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992; 99:19–28.

2. Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991; 109:77–83.

3. Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol. 1993; 111:62–5.

4. Sihota R, Sony P, Gupta V, et al. Comparing glaucomatous optic neuropathy in primary open angle and chronic primary angle clo-sure glaucoma eyes by optical coherence tomography. Ophthalmic Physiol Opt. 2005; 25:408–15.

5. Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989; 107:453–64.

6. Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990; 300:5–25.

7. Zeimer R, Asrani S, Zou S, et al. Quantitative detection of glau-comatous damage at the posterior pole by retinal thickness mapping. A pilot study. Ophthalmology. 1998; 105:224–31.
8. Burgansky-Eliash Z, Wollstein G, Chu T, et al. Optical coherence tomography machine learning classifiers for glaucoma detection: A preliminary study. Invest Ophthalmol Vis Sci. 2005; 46:4147–52.

9. Huang ML, Chen HY. Development and comparison of automated classifiers for glaucoma diagnosis using Stratus optical coherence tomography. Invest Ophthalmol Vis Sci. 2005; 46:4121–9.

10. Manassakorn A, Nouri-Mahdavi K, Caprioli J. Comparison of reti-nal nerve fiber layer thickness and optic disk algorithms with opti-cal coherence tomography to detect glaucoma. Am J Ophthalmol. 2006; 141:105–15.

11. Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measure-ments for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005; 139:44–55.

12. Parikh RS, Parikh S, Sekhar GC, et al. Diagnostic capability of op-tical coherence tomography (stratus OCT 3) in early glaucoma. Ophthalmology. 2007; 114:2238–43.

13. Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical co-herence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006; 142:576–82.

14. Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sen-sitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000; 41:1774–82.
15. Parikh RS, Parikh SR, Thomas R. Diagnostic capability of macular parameters of Stratus OCT 3 in detection of early glaucoma. Br J Ophthalmol. 2010; 94:197–201.

16. Wollstein G, Ishikawa H, Wang J, et al. Comparison of three opti-cal coherence tomography scanning areas for detection of glau-comatous damage. Am J Ophthalmol. 2005; 139:39–43.

17. Nakatani Y, Higashide T, Ohkubo S, et al. Evaluation of macular thickness and peripapillary retinal nerve fiber layer thickness for detection of early glaucoma using spectral domain optical coher-ence tomography. J Glaucoma. 2011; 20:252–9.

18. Na JH, Sung KR, Baek S, et al. Macular and retinal nerve fiber lay-er thickness: which is more helpful in the diagnosis of glaucoma. Invest Ophthalmol Vis Sci. 2011; 52:8094–101.

19. Leung CK, Chan WM, Yung WH, et al. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology. 2005; 112:391–400.
20. Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009; 50:3432–7.

21. Han IC, Jaffe GJ. Evaluation of artifacts associated with macular spectral-domain optical coherence tomography. Ophthalmology. 2010; 117:1177–1189.e4.

22. Asrani S, Challa P, Herndon L, et al. Correlation among retinal thickness, optic disc, and visual field in glaucoma patients and sus-pects: a pilot study. J Glaucoma. 2003; 12:119–28.

23. Greenfield DS, Bagga H, Knighton RW. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol. 2003; 121:41–6.

24. Asrani S, Rosdahl JA, Allingham RR. Novel software strategy for glaucoma diagnosis: asymmetry analysis of retinal thickness. Arch Ophthalmol. 2011; 129:1205–11.
25. Ojima T, Tanabe T, Hangai M, et al. Measurement of retinal nerve fiber layer thickness and macular volume for glaucoma detection using optical coherence tomography. Jpn J Ophthalmol. 2007; 51:197–203.

26. Tan O, Li G, Lu AT, et al. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008; 115:949–56.

27. Ishikawa H, Stein DM, Wollstein G, et al. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005; 46:2012–7.

28. Wagner-Schuman M, Dubis AM, Nordgren RN, et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011; 52:625–34.

29. Kashani AH, Zimmer-Galler IE, Shah SM, et al. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol. 2010; 149:496–502.

30. Ooto S, Hangai M, Sakamoto A, et al. Three-dimensional profile of macular retinal thickness in normal Japanese eyes. Invest Ophthalmol Vis Sci. 2010; 51:465–73.

Go to : 
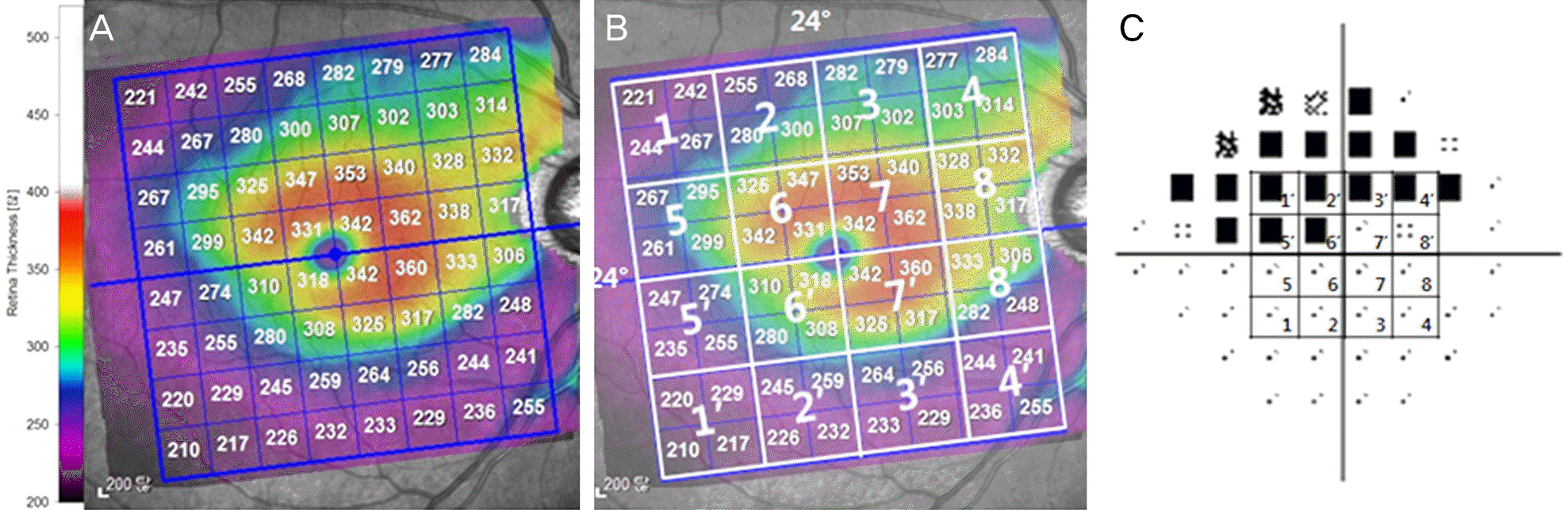 | Figure 1.The mean retinal thickness mapping corresponding to the visual field sensitivity. (A) A macular thickness map yielded by posterior pole asymmetry analysis of spectral domain optical coherence tomography is divided into 64 squares centered on the fovea. (B) We divided both the superior and inferior hemifields into 8 sectorsparts based on the horizontal raphe. Retinal thickness values of 4 ad-jacent square cells in the 8 χ8 grid of the posterior pole thickness map were averaged on the mean retinal thickness value. (C) Among the 52 test points of the central 24-2 pattern, the central 4 ×4 points corresponding to the 24° χ 24° posterior pole thickness map of the SPECTRALIS spectral-domain optical coherence tomography (SD-OCT) were considered. Same number between (B) and (C) indicated a pair of sector to undergo statistical analysis. |
Table 1.
Demographic characteristics of study participants
| Normal (n = 30) | Glaucoma (n = 30) | p-value | |
|---|---|---|---|
| Age (years) | 52.03 ± 3.98 | 58.80 ± 4.12 | 0.55* |
| Sex (M:F) | 13:17 | 20:10 | 0.07† |
| Axial length (mm) | 23.83 ± 0.46 | 24.10 ± 0.58 | 0.30* |
| Posterior pole retinal thickness (μm) | 294.15 ± 33.98 | 278.3 ± 41.03 | <0.01* |
| Global index of standard automated perimetry | |||
| MD (dB) | 0.34 ± 0.91 | -3.18 ± 5.89 | 0.03* |
| PSD (dB) | 1.36 ± 0.10 | 3.92 ± 1.86 | <0.01* |
Table 2.
Mean retinal thickness of normal and glaucomatous eyes and cut off values in 16 sectors as well as average determined by SD-OCT
| Sector |
Control |
Glaucoma |
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (μm) | Range (μm) | <5% (μm) | <1% (μm) | Mean ± SD (μm) | p-value* | ||
| Superior | 1 | 242.61 ± 8.57 | 230.25-254.50 | 224.08 | 217.25 | 231.76 ± 11.90 | <0.01 |
| 2 | 273.20 ± 9.99 | 259.50-288.75 | 251.66 | 248.75 | 258.14 ± 15.43 | <0.01 | |
| 3 | 292.49 ± 10.23 | 277.75-311.00 | 277.79 | 269.00 | 273.30 ± 18.02 | <0.01 | |
| 4 | 307.06 ± 12.75 | 287.25-334.00 | 287.25 | 284.50 | 274.10 ± 22.34 | <0.01 | |
| 5 | 268.84 ± 12.55 | 244.50-288.25 | 242.36 | 237.00 | 256.62 ± 13.43 | <0.01 | |
| 6 | 322.70 ± 13.50 | 294.50-338.00 | 293.55 | 291.75 | 306.86 ± 15.70 | <0.01 | |
| 7 | 336.46 ± 13.72 | 305.75-344.25 | 309.03 | 308.25 | 323.58 ± 18.77 | 0.04 | |
| 8 | 314.27 ± 13.20 | 289.75-333.25 | 289.90 | 282.00 | 299.94 ± 35.23 | 0.04 | |
| Inferior | 1’ | 268.50 ± 9.89 | 227.50-321.25 | 249.15 | 245.00 | 224.07 ± 15.32 | <0.01 |
| 2’ | 269.24 ± 11.09 | 255.00-305.25 | 250.49 | 243.75 | 246.82 ± 14.75 | <0.01 | |
| 3’ | 287.96 ± 12.54 | 268.25-314.75 | 265.64 | 261.75 | 261.62 ± 19.69 | <0.01 | |
| 4’ | 304.00 ± 15.00 | 251.50-324.75 | 279.30 | 251.50 | 272.17 ± 27.47 | <0.01 | |
| 5’ | 269.78 ± 16.58 | 249.50-336.25 | 244.51 | 234.75 | 254.58 ± 13.72 | <0.01 | |
| 6’ | 324.26 ± 14.19 | 295.00-344.50 | 293.81 | 290.00 | 304.43 ± 19.28 | <0.01 | |
| 7’ | 331.91 ± 14.12 | 302.00-352.00 | 305.36 | 302.00 | 319.86 ± 21.19 | <0.01 | |
| 8’ | 313.50 ± 15.75 | 287.75-346.50 | 287.80 | 283.50 | 292.54 ± 20.52 | <0.01 | |
Table 3.
Agreements of abnormal judgment between visual field sensitivity and mean retinal thickness, measurement in 16 tested sectors
| Sector |
Criteria A* |
Criteria B† |
|||
|---|---|---|---|---|---|
| Kappa value | p-value | Kappa value | p-value | ||
| Superior | 1 | -0.320 | 0.028 | 0.000 | 1.000 |
| 2 | -0.401 | 0.002 | -0.302 | 0.015 | |
| 3 | -0.418 | 0.014 | -0.363 | 0.015 | |
| 4 | 0.053 | 0.593 | 0.094 | 0.397 | |
| 5 | 0.02 | 0.894 | -0.064 | 0.506 | |
| 6 | 0.429 | 0.014 | 0.429 | 0.014 | |
| 7 | 0.132 | 0.356 | 0.132 | 0.356 | |
| 8 | 0.173 | 0.092 | 0.173 | 0.092 | |
| Inferior | 1’ | -0.068 | 0.023 | -0.068 | 0.023 |
| 2’ | 0.110 | 0.351 | 0.187 | 0.170 | |
| 3’ | 0.233 | 0.102 | 0.268 | 0.070 | |
| 4’ | 0.170 | 0.190 | 0.444 | 0.014 | |
| 5’ | 0.379 | 0.037 | 0.380 | 0.008 | |
| 6’ | 0.286 | 0.088 | 0.000 | 1.000 | |
| 7’ | -0.119 | 0.377 | -0.105 | 0.513 | |
| 8’ | -0.017 | 0.900 | 0.020 | 0.894 | |
Table 4.
The sensitivity and specificity of diagnostic criteria A and B of retinal thickness measurements to detect abnormal visual field sensitivity in 16 tested sectors




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download