Abstract
Purpose
To determine the surface roughness of cosmetic and conventional contact lenses (CLs) and their susceptibility to bacterial adhesion.
Methods
Concave surface roughness of cosmetic and conventional hydrogel (Etafilcon A) CLs was measured by atomic force microscopy (AFM) and scanning electron microscopy (SEM). In particular, the surface of the color tinted area of cosmetic CLs was measured. CLs were immersed into a bacterial solution of Pseudomonas aeruginosa for 1, 12, or 24 hours and culture of P. aeruginosa that had adhered to the CLs was performed.
Results
Concave surface roughness of cosmetic CLs significantly increased compared with conventional CLs by AFM (p < 0.05). Bacterial colony formation of P. aeruginosa adhering to cosmetic CLs within one hour significantly increased compared with conventional CLs (p = 0.047). Adhesions of P. aeruginosa to CLs within one hour was found to correlate significantly with the surface roughness of CL (r > 0.9, p < 0.05). By SEM, P. aeruginosa had adhered to the color-tinted area more than to the non-color-tinted area of cosmetic CLs.
Conclusions
Surface of cosmetic CLs was significantly rougher and initial adhesion of bacteria was higher to cosmetic CLs than to conventional CLs. In particular, an increased number of bacteria was found to be adhered to the color-tinted area of cosmetic CLs. Initial bacterial adhesion is important because it is the first stage of bacterial attachment process to any surface. After then, the adherent bacteria can progress to form a biofilm. Increased surface roughness of CLs contributes to opportunities for the CL to come into contact with bacteria, and thus, initial bacterial adhesion increases. In this study, it is clear that cosmetic CLs are more vulnerable to bacterial adhesion. To avoid serious complications, such as bacterial keratitis, the manufacturing process for smoothing and treating the surface in order to inhibit bacterial adhesion should be developed in the future.
Go to : 
References
1. Efron N, Morgan PB, Woods CA. International survey of contact lens prescribing for extended wear. Optom Vis Sci. 2012; 89:122–9.

2. Efron N, Morgan PB, Woods CA. Survey of contact lens prescribing to infants, children, and teenagers. Optom Vis Sci. 2011; 88:461–8.

3. Morgan PB, Efron N, Woods CA. Determinants of the frequency of contact lens wear. Eye Contact Lens. 2013; 39:200–4.

4. Efron N, Morgan PB, Woods CA. An international survey of daily disposable contact lens prescribing. Clin Exp Optom. 2013; 96:58–64.
5. Park YM, Hahn TW, Choi SH, et al. Acanthamoeba Keratitis Related to Cosmetic Contact Lenses. J Korean Ophthalmol Soc. 2007; 48:991–4.
6. Song JS, Lee H, Kim JW, et al. The effects of cheap tinted contact lenses on corneal swelling and ocular surface inflammation. J Korean Ophthalmol Soc. 2008; 49:1888–93.

7. Lim TH, Lee JR, Choi KY, et al. Corneal melting And descemetocele resulting from noninfectious keratitis related to the cosmetic contact lenses. J Korean Ophthalmol Soc. 2009; 50:774–8.

8. Park SJ, Lee SM, Kim MK, et al. Cosmetic contact lens-related complications: 9 cases. J Korean Ophthalmol Soc. 2009; 50:927–35.

9. Sauer A, Bourcier T. Microbial keratitis as a foreseeable complication of cosmetic contact lenses: a prospective study. Acta Ophthalmol. 2011; 89:e439–42.

10. Steinemann TL, Fletcher M, Bonny AE, et al. Over-the-counter decorative contact lenses: Cosmetic or Medical Devices? A Case Series. Eye Contact Lens. 2005; 31:194–200.

11. Steinemann TL, Pinninti U, Szczotka LB, et al. Ocular complications associated with the use of cosmetic contact lenses from unlicensed vendors. Eye Contact Lens. 2003; 29:196–200.

12. McKelvie J, Patel D, McGhee C. Cosmetic contact lens-related Acanthamoeba keratitis. Clin Experiment Ophthalmol. 2009; 37:419–20.
13. Williams D. Great expectations and the grapes of wrath: contamination of contact lenses. Med Device Technol. 1999; 10:10–3.
14. Giraldez MJ, Resua CG, Lira M, et al. Contact lens hydrophobicity and roughness effects on bacterial adhesion. Optom Vis Sci. 2010; 87:E426–31.

15. Burnham GW, Cavanagh HD, Robertson DM. The impact of cellular debris on Pseudomonas aeruginosa adherence to silicone hydrogel contact lenses and contact lens storage cases. Eye Contact Lens. 2012; 38:7–15.

16. Dutta D, Cole N, Willcox M. Factors influencing bacterial adhesion to contact lenses. Mol Vis. 2012; 18:14–21.
17. Vijay AK, Zhu H, Ozkan J, et al. Bacterial adhesion to unworn and worn silicone hydrogel lenses. Optom Vis Sci. 2012; 89:1095–106.

18. Willcox MD. Microbial adhesion to silicone hydrogel lenses: a review. Eye Contact Lens. 2013; 39:61–6.
20. Robertson DM. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye Contact Lens. 2013; 39:67–72.

21. Bailey CS. A review of relative risks associated with four types of contact lenses. Cornea. 1990; 9(Suppl 1):S59–61. discussion S62-3.

22. Willcox MD, Harmis N, Cowell , et al. Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials. 2001; 22:3235–47.

23. Bruinsma GM, van der Mei HC, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001; 22:3217–24.

24. McGlinchey SM, McCoy CP, Gorman SP, Jones DS. Key biological issues in contact lens development. Expert Rev Med Devices. 2008; 5:581–90.

25. Loyola-Rodriguez JP, Zavala-Alonso V, Reyes-Vela E, et al. Atomic force microscopy observation of the enamel roughness and depth profile after phosphoric acid etching. J Electron Microsc (Tokyo). 2010; 59:119–25.

26. Lira M, Santos L, Azeredo J, et al. Comparative study of silicone-hydrogel contact lenses surfaces before and after wear using atomic force microscopy. J Biomed Mater Res B Appl Biomater. 2008; 85:361–7.

27. Giraldez MJ, Serra C, Lira M, et al. Soft contact lens surface profile by atomic force microscopy. Optom Vis Sci. 2010; 87:E475–81.

28. Desrousseaux C, Sautou V, Descamps S, Traore O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J Hosp Infect. 2013; 85:87–93.

29. Mueller RF, Characklis WG, Jones WL, Sears JT. Characterization of initial events in bacterial surface colonization by two Pseudomonas species using image analysis. Biotechnol Bioeng. 1992; 39:1161–70.
30. Chen L, Wen YM. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011; 3:66–73.

31. Fletcher EL, Weissman BA, Efron N, et al. The role of pili in the attachment of Pseudomonas aeruginosa to unworn hydrogel contact lenses. Curr Eye Res. 1993; 12:1067–71.
32. Evans DJ, Fleiszig SM. Microbial keratitis: could contact lens material affect disease pathogenesis. Eye Contact Lens. 2013; 39:73–8.
Go to : 
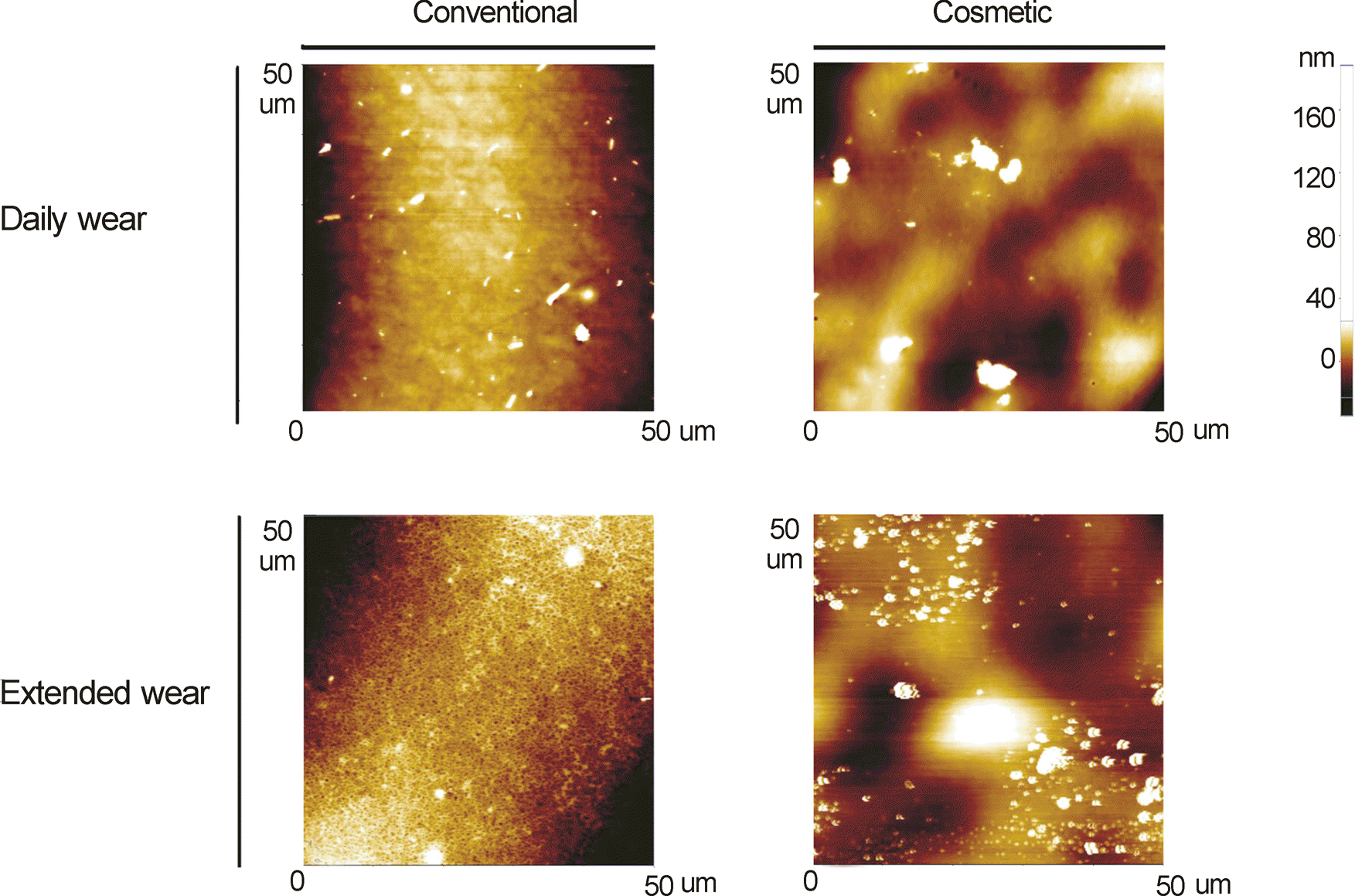 | Figure 2.Atomic force micrographs of surface of different contact lenses (CLs) on the concave side. The surface topography of four different CLs was obtained through atomic force microscopy (AFM) analysis over a 2500 μm2 surface area on the concave side. Especially measuring cosmetic CLs, color tinted area was contained. (A) Conventional CL - daily wear. (B) Cosmetic CL - daily wear. (C) Conventional CL - extended wear. (D) Cosmetic CL - extended wear. |
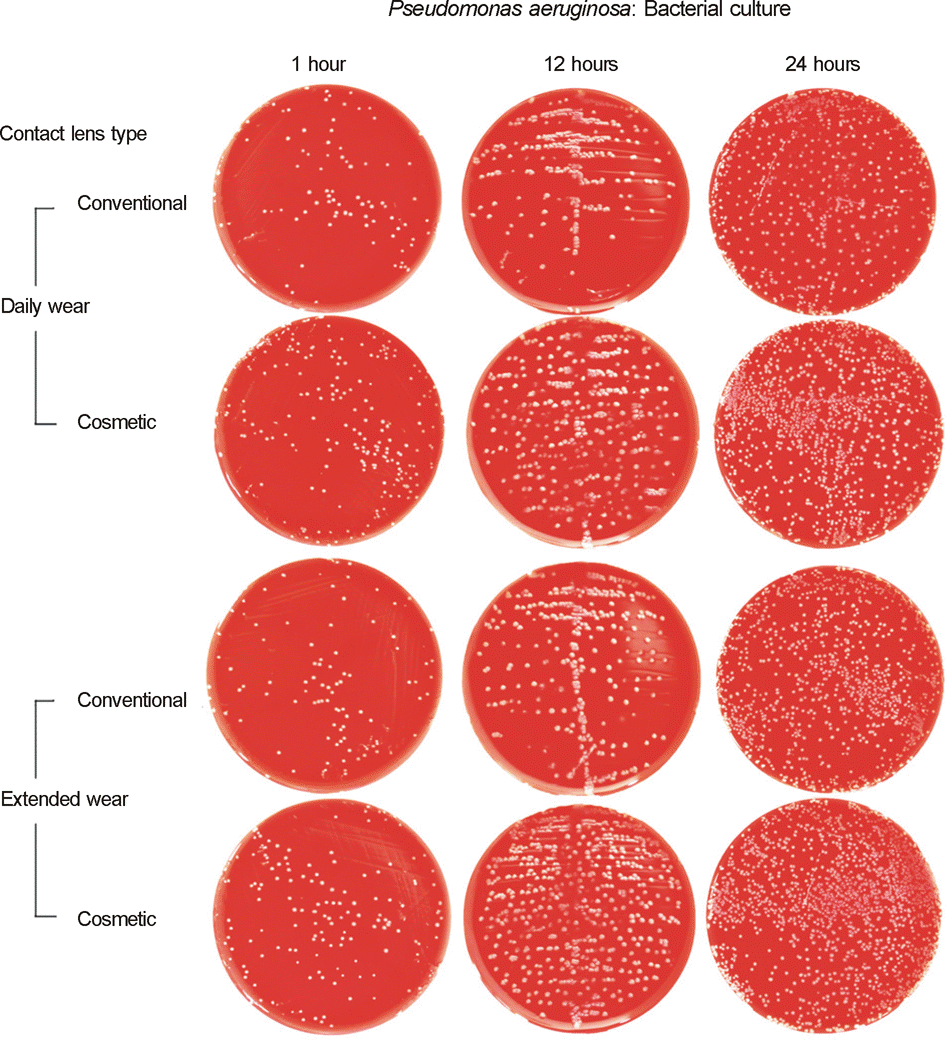 | Figure 3.Bacterial culture of Pseudomonas aeruginosa adhering to contact lenses after immersing the contact lenses (CLs) into bacterial solutions for 1 hour, 12 hours, or 24 hours, using right angle streaking method. (The bacterial solution for P. aeruginosa adhesion to CLs during 24 hours was diluted to 1:4, compared to others for 1 and 12 hours.) |
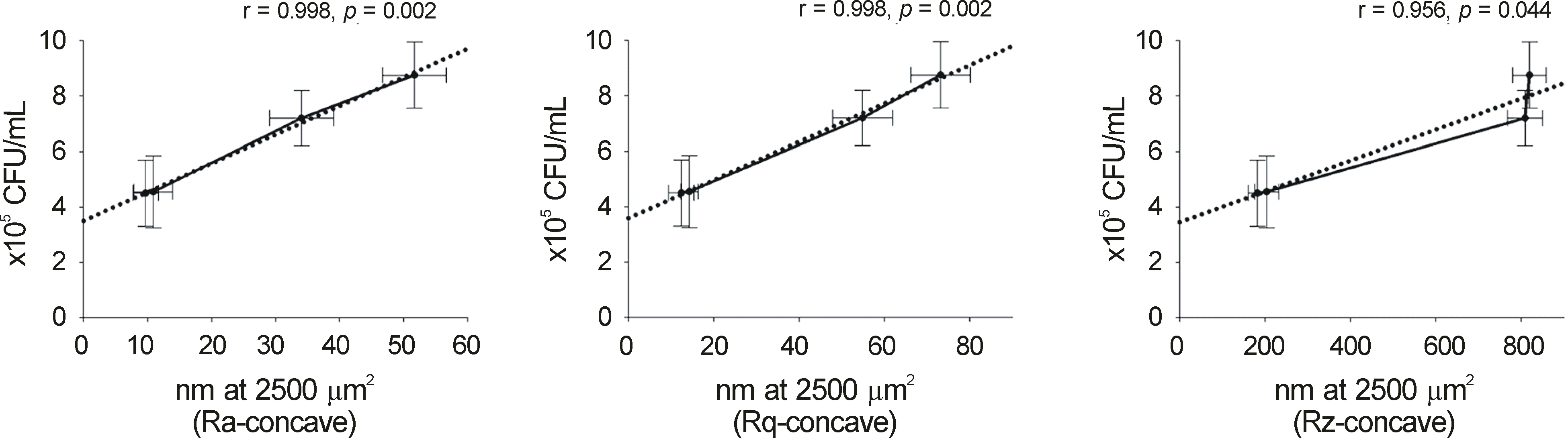 | Figure 4.Correlation of surface roughness and initial (1 hour) number of Pseudomonas aeruginosa adhering to contact lenses (CLs) (r: Pearson correlation coefficient −1 ≤ r ≤1 by the Pearson correlation test). |
 | Figure 5.Scanning electron microscopic photographs of cosmetic contact lens surface and its Pseudomonas aeruginosa adhesions. Cosmetic contact lenses (CLs) were immersed into P. aeruginosa for 24 hours. After the CLs were washed and processed for scanning electron microscopy (SEM). Then, the microscopic photographs of concave side of CLs were taken from SEM. (A) Color tinted area of cosmetic CL was rougher than non-color tinted(× 1000). (B) Black lined square of Fig. A was observed at higher magnification (×1000). (C) P. aeruginosa (white arrow head) was adhered to irregular surface of cosmetic CL. Especially, more bacterial adhesions were found to color tinted area (×2000). (D) P. aeruginosa adhesion to surface of CL was observed at high magnification (×10000). (N: non-color tinted area, C: color tinted area, white arrow head: P aeruginosa adhesions). |
Table 1.
Contact lens (CL) used in the study
Table 2.
Surface roughness of contact lenses (CLs) measured by atomic force microscope (AFM) on concave side (nm at 2500 μm2)
Table 3.
Comparison of concave surface roughness between different contact lenses (CLs)
(a) Conventional vs Cosmetic (nm at 2500 μm2)
| Average roughness (Ra) | p-value | Root mean square roughness (Rq) | p-value | Ten points mean height roughness (Rz) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Conventional | Cosmetic | Conventional | Cosmetic | Conventional | Cosmetic | |||
| 10.29 ± 0.85 | 42.86 ± 12.49 | 0.067 | 13.33 ± 1.33 | 64.00 ± 12.93 | 0.031* | 192.81 ± 15.11 | 814.21 ± 7.26 | 0.002† |
| Values are presented as mean ± SD. | ||||||||
| (b) Daily wear (DW) vs Extended wear (EW) (nm at 2500 μm2) | ||||||||
Table 4.
Bacterial culture of Pseudomonas aeruginosa adhering to different contact lenses (CLs), after immersing the CLs into bacterial solutions for 1 hour, 12 hours, or 24 hours (×105 CFU/mL)
Table 5.
Comparison of Pseudomonas aeruginosa adhering to different contact lenses (CLs), after immersing the CLs into bacterial solutions for 1 hour, 12 hours, or 24 hours
(a) Conventional vs Cosmetic (×105 CFU/mL)
| Immersing time - 1 hour | p-value | Immersing time - 12 hours | p-value | Immersing time - 24 hours | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Conventional | Cosmetic | Conventional | Cosmetic | Conventional | Cosmetic | |||
| 4.53 ± 0.04 | 7.98 ± 1.10 | 0.047* | 12.68 ± 0.88 | 17.55 ± 3.11 | 0.167 | 94.50 ± 7.50 | 117.50 ± 21.92 | 0.295 |
| Values are presented as mean ± SD. | ||||||||
| (b) Daily wear (DW) vs Extended wear (EW) (×105 CFU/mL) | ||||||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download