Abstract
Purpose
To evaluate the long-term results of intravitreal bevacizumab injection for macular edema secondary to branch retinal vein occlusion (BRVO).
Methods
Fifty-six eyes with macular edema secondary to BRVO were treated with intravitreal bevacizumab injection. They were classified into two groups, one group that received three initial intravitreal bevacizumab loadings at monthly in-tervals and a second group that received only one initial injection. In the two groups, additive injection was performed at recurrence. The best corrected visual acuity (BCVA), central macular thickness (CMT) and retinal ischemic change was analyzed for more than 12 months postoperatively.
Results
After 12 months of follow-up, mean BCVA improved and mean CMT reduced significantly in both groups (p < 0.05). However, the range of BCVA improvement and CMT reduction was wider in the three-injection group than in the sin-gle-injection group. Fluorescein angiography revealed posterior retinal ischemic changes; bevacizumab didn’t seem to ag-gravate the ischemic change. No drug-related ocular or systemic side effects were observed in the follow-up period after intravitreal bevacizumab treatment except subconjunctival hemorrhage and a mild increase of intraocular pressure.
Go to : 
References
1. Ehlers JP, Decroos FC, Fekrat S. Intravitreal bevacizumab for macular edema secondary to branch retinal vein occlusion. Retina. 2011; 31:1856–62.

2. Ravena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007; 27:419–25.
3. The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984; 98:271–82.
4. Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am J Ophthalmol. 2005; 140:695–702.

5. Russo V, Barone A, Conte E, et al. Bevacizumab compared with macular laser grid photocoagulation for cystoid macular edema in branch retinal vein occlusion. Retina. 2009; 29:511–5.

6. Cekiç O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005; 25:851–5.

7. Chen SD, Sundaram V, Lochhead J, Patel CK. Intravitreal triamcinolone triamcinolone for the treatment of ischemic macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2006; 141:876–83.
8. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350:2335–42.

9. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.

10. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

11. Funk M, Kriechbaum K, Prager F, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Invest Ophthalmol Vis Sci. 2009; 50:1025–32.

12. Rensch F, Jonas JB, Spandau UH. Early intravitreal bevacizumab for non-ischaemic branch retinal vein occlusion. Ophthalmologica. 2009; 223:124–7.

13. Kim YG, Kim ES, Kim MS, et al. Early and late intravitreal bevacizumab injection in macular edema due to branch retinal vein occlusion. J Korean Ophthalmol Soc. 2009; 50:1527–30.

14. Yasuda S, Kondo M, Kachi S, et al. Rebound of macular edema after intravitreal bevacizumab therapy in eyes with macular edema secondary to branch retinal vein occlusion. Retina. 2011; 31:1075–82.

15. Wu L, Arevalo JF, Berrocal MH, et al. Comparison of two doses of intravitreal bevacizumab as primary treatment for macular edema secondary to branch retinal vein occlusions: results of the Pan American Collaborative Retina Study Group at 24 months. Retina. 2009; 29:1396–403.
16. Kriechbaum K, Michels S, Prager F, et al. Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol. 2008; 92:518–22.

17. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009; 93:452–6.
18. Siegel RA, Dreznik A, Mimouni K, et al. Intravitreal bevacizumab treatment for macular edema due to branch retinal vein occlusion in a clinical setting. Curr Eye Res. 2012; 37:823–9.

19. Lee SW, Kim MS, Kim ES, et al. Long-term results of intravitreal bevacizumab injection for macular edema : retinal vein obstruction and diabetic retinopathy. J Korean Ophthalmol Soc. 2009; 50:211–8.
20. Lee YS, Kim MS, Yu SY, Kwak HW. Two-year results of intravitreal bevacizumab injection in retinal vein occlusion. J Korean Ophthalmol Soc. 2011; 52:1039–47.

21. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008; 146:508–12.

22. Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005; 46:726–33.

23. Badalà F. The treatment of branch retinal vein occlusion with bevacizumab. Curr Opin Ophthalmol. 2008; 19:234–8.

24. Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms: ETDRS report number 11. Ophthalmol. 1991; 98(5 Suppl):807–22.
25. Blodi BA, Domalpally A, Scott IU, et al. SCORE Study Research Group. Standard care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study for evaluation of stereroscopic color fundus photographs and fluorescein angiograms: SCORE Study report 9. Arch Ophthalmol. 2010; 128:1140–5.
26. Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007; 27:419–25.

27. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–9.

28. Jaissle GB, Ziemssen F, Petermeier K, et al. Bevacizumab for treatment of macular edema secondary to retinal vein occlusion. Ophthalmologe. 2006; 103:471–5.
29. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1102–12.
30. Pournaras JA, Nguyen C, Vaudaux JD, et al. Treatment of central retinal vein occlusion-related macular edema with intravitreal bevacizumab (avastin): preliminary results. Klin Monbl Augenheilkd. 2008; 225:397–400.

31. Höh AE, Schaal KB, Scheuerle A, et al. OCT-guided reinjection of 2.5 mg bevacizumab for treating macular edema due to retinal vein occlusion. Ophthalmologe. 2008; 105:1121–6.
33. Kim KS, Chang HR, Song S. Ischaemic change after intravitreal bevacizumab (Avastin) injection for macular oedema secondary to non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2008; 86:925–7.

34. Sabet-Peyman EJ, Heussen FM, Thorne JE, et al. Progression of macular ischemia following intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2009; 40:316–8.

35. Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013; 120:795–802.

36. Terui T, Kondo M, Sugita T, et al. Changes in arears of capillary nonperfusion after intravitreal injection of bevacizumab in eyes with branch retinal vein occlusion. Retina. 2011; 31:1068–74.
37. Mansour AM, Bynoe LA, Welch JC, et al. Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmol. 2010; 88:730–5.

38. SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study report 6. Arch Ophthalmol. 2009; 127:1115–28.
Go to : 
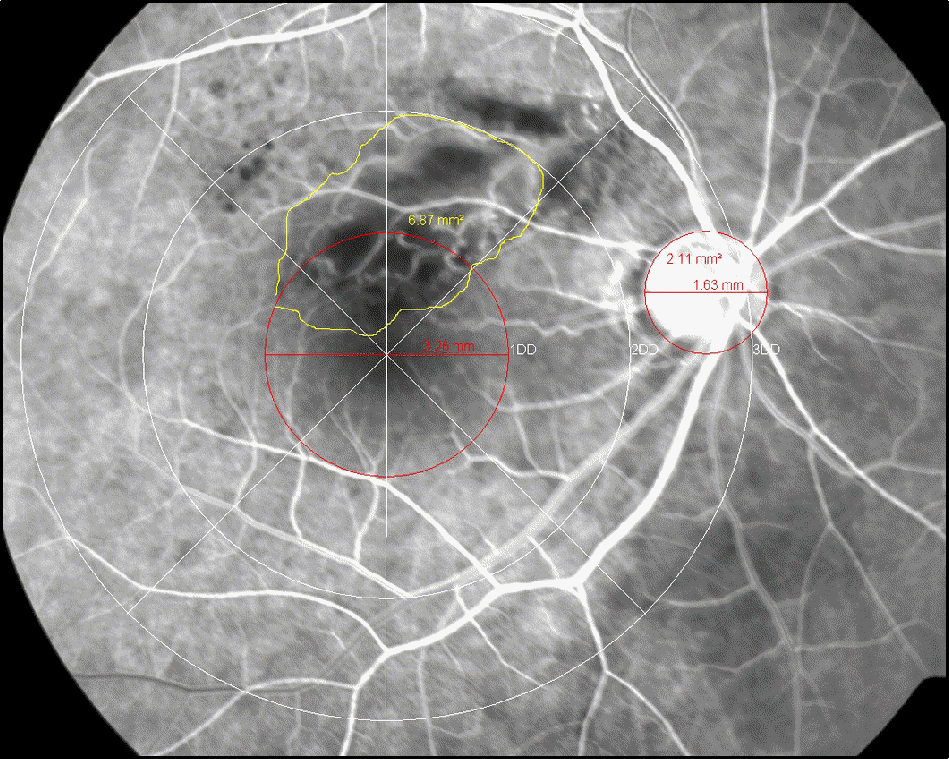 | Figure 1.Measurement of posterior nonperfusion area in a FA photograph of a retina with BRVO. There are three concentric circles centered on the macula. The radius of the innermost circle corresponds to 1DD; the radius of the second circle, to 2DD. The inner field surrounding this second circle is the so-called field 2F in the ETDRS FA grading protocol. The area of posterior capillary nonperfusion in the field 2F is out-lined by the use of the area measurement tool of a digital system. Blocked fluorescence lesions because of retinal hem-orrhages were distinguished from nonperfusion by comparing the FA and fundus images. The area of nonperfusion is ex-pressed relative to the DA. |
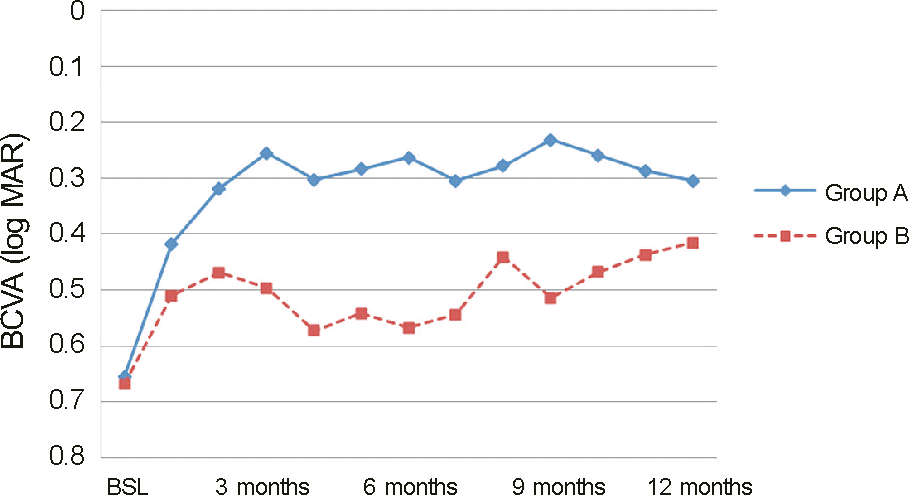 | Figure 2.Change in best corrected visual acuity (BCVA) from baseline (BSL) to month 12. There is significant improvement in BCVA as compared with BSL values in both group A and B at every follow-up visit. But the outcomes of group A are superior to group B’s at every visit. |
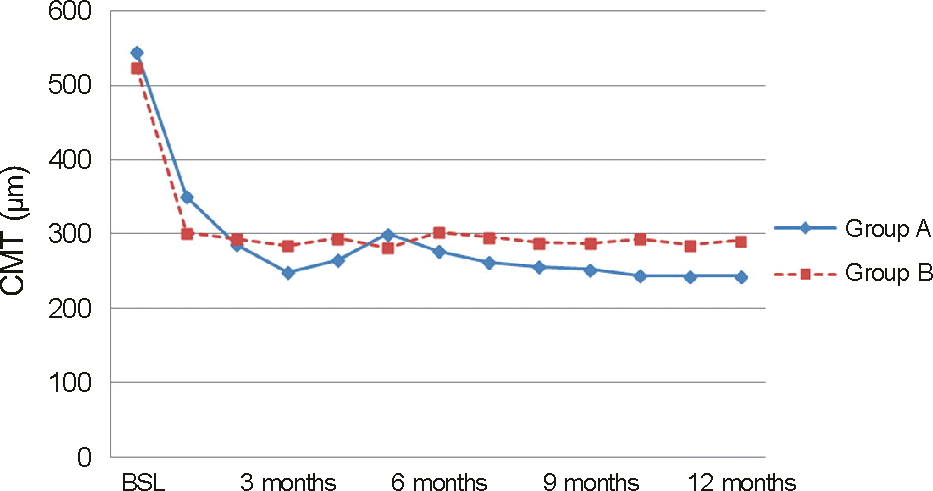 | Figure 3.Change in central macular thickness (CMT) from baseline (BSL) to month 12. There is significant reduction in CMT as compared with BSL values in both group A and B at every follow-up visit. But the outcomes of group A are superior to group B’s at every visit except month 1 and 5. |
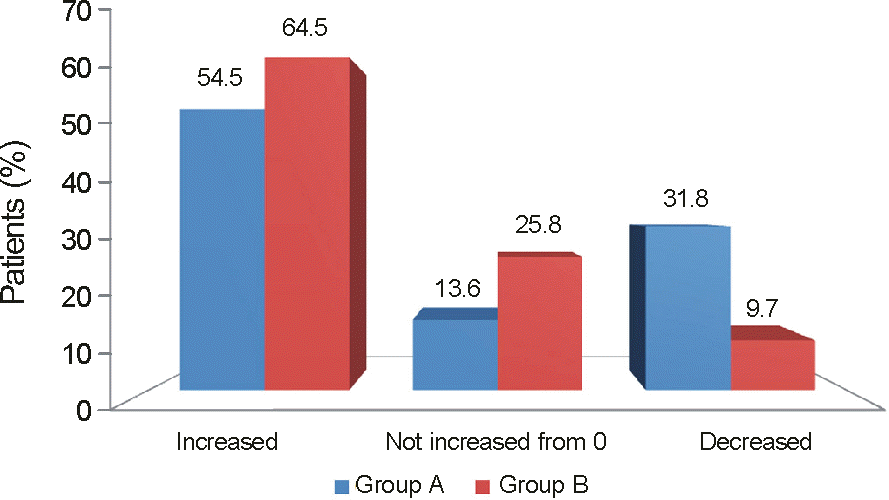 | Figure 4.The percentages of patients in each treatment group who showed an increase, decrease or no change from 0 in retinal nonperfusion between baseline and the month 12 end point. |
Table 1.
Demographics of patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download