Abstract
Purpose
To evaluate the success and complication rates of triple procedure, including pterygium excision, marginal amniotic membrane insertion beneath the conjunctiva, and limbal-conjunctival autograft in pterygium surgery.
Methods
We conducted a retrospective study on 45 eyes of 45 patients who underwent pterygium surgery between August 2011 and October 2012. After pterygium excision, amniotic membrane was placed beneath the conjunctiva along the margin of the exposed sclera followed by a limbal conjunctival autograft. Success rates, intraoperative and post-operative complications were evaluated.
Results
Forty-three eyes with primary pterygium and 2 eyes with recurrent pterygium were enrolled in the present study. The mean age of the patients was 59.87 ± 14.30 years with a mean follow-up of 12.9 ± 4.6 months. There were no complications during surgery. Early postoperative complications included partial wound dehiscence in 1 eye and a simple conjunctival cyst on the autografted conjunctiva in the another eye. No clinically significant recurrence (G2, G3) was noted during the observational periods. Thirty-nine (86.7%) and 6 (13.3%) eyes were graded as G0 and G1, respectively.
Conclusions
Our surgical technique not only has the benefits of the limbal conjunctival autograft acting as a barrier against fibrovascular invasion of the cornea and supplying stem cells to the corneal epithelium but also has antiangiogenic effects of amniotic membrane with minimal use. In addition, this technique is a safe surgical method in primary and recurrent pterygium.
Go to : 
References
1. Lee JS, Lee SW, Lee SJ, Kim NM. Effects of cyclosporin on pterygium fibroblasts. J Korean Ophthalmol Soc. 2012; 53:466–72.

2. Kim JW, Ahn J, Kook KH, Yang H. Recurrence rates of conjunctival autograft transplantation with aminiotic membrane transplantation in primary pterygium surgery. J Korean Ophthalmol Soc. 2011; 52:163–8.

3. Coroneo MT, Di Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 1999; 10:282–8.

4. Yoon KC, Mun GH, Kim SD, et al. Prevalence of eye diseases in South Korea: data from the Korea national health and nutrition examination survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–33.

5. Gu BY, Lee SB. Effects of temporary amniotic membrane patch after surgical excision of primary pterygium. J Korean Ophthalmol Soc. 2012; 53:749–60.

6. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997; 104:974–85.

7. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphol-ogy on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997; 115:1235–40.

8. Li M, Zhu M, Yu Y, et al. Comparison of conjunctival autograft transplantation and amniotic membrane transplantation for pterygium: a meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2012; 250:375–81.

9. Panda A, Das GK, Tuli SW, Kumar A. Randomized trial of intraoperative mitomycin C in surgery for pterygium. Am J Ophthalmol. 1998; 125:59–63.

10. Bae SG, Kim JK, Lee JK, Park DJ. The effectiveness of mitomycin C on pterygium surgery with amniotic membrane transplantation. J Korean Ophthalmol Soc. 2012; 53:200–7.

11. Cho JW, Chung SH, Seo KY, Kim EK. Conjunctival mini-flap technique and conjunctival autotransplantation in pterygium surgery. J Korean Ophthalmol Soc. 2005; 46:1471–7.
12. Todani A, Melki SA. Pterygium: current concepts in pathogenesis and treatment. Int Ophthalmol Clin. 2009; 49:21–30.
13. Al Fayez MF. Limbal-conjunctival vs conjunctival autograft transplant for recurrent pterygia: a prospective randomized controlled trial. JAMA Ophthalmol. 2013; 131:11–6.
14. Küçükerdönmez C, Akova YA, Altinörs DD. Vascularization is more delayed in amniotic membrane graft than conjunctival autograft after pterygium excision. Am J Ophthalmol. 2007; 143:245–9.

15. Ma DH, See LC, Liau SB, Tsai RJ. Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 2000; 84:973–8.

16. Kim HJ, Lee SB. Comparison of permanent amniotic membrane transplantation and temporary amniotic membrane patch after primary pterygium excision. J Korean Ophthalmol Soc. 2012; 53:1236–46.

17. Hao Y, Ma DH, Hwang DG, et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000; 19:348–52.

18. Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000; 20:173–7.

19. Tananuvat N, Martin T. The results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograft. Cornea. 2004; 23:458–63.

20. Yang SF, Lin CY, Yang PY, et al. Increased expression of gelatinase (MMP-2 and MMP-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Invest Ophthalmol Vis Sci. 2009; 50:4588–96.

21. Chan CM, Chew PT, Alsagoff Z, et al. Vascular patterns in pterygium and conjunctival autografting: a pilot study using indocyanine green anterior segment angiography. Br J Ophthalmol. 2001; 85:350–3.

22. Mutlu FM, Sobaci G, Tatar T, Yildirim E. A comparative study of recurrent pterygium surgery: limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology. 1999; 106:817–21.

23. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphol-ogy on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997; 115:1235–40.

24. Barraquer JI, Binder PS, Buxton JN. Etiology and treatment of pterygium; Symposium on Medical and Surgical Disease of the Cornea. Transactions of the New Orleans Academy of Ophthalmology. Louids: St. Mosby;1980. p. 167–78.
25. Luanratanakorn P, Ratanapakorn T, Suwan-Apichon O, Chuck RS. Randomised controlled study of conjunctival autograft versus amniotic membrane graft in pterygium excision. Br J Ophthalmol. 2006; 90:1476–80.

26. Shimazaki J, Kosaka K, Shimmura S, Tsubota K. Amniotic membrane transplantation with conjunctival autograft for recurrent pterygium. Ophthalmology. 2003; 110:119–24.

27. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989; 96:709–22. discussion 722-3.

28. Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999; 18:73–9.

29. Khodadoust AA, Silverstein AM, Kenyon DR, Dowling JE. Adhesion of regenerating corneal epithelium. The role of basement membrane. Am J Ophthalmol. 1968; 65:339–48.
30. Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999; 40:878–86.
31. Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995; 267:891–3.

32. Casey ML, MacDonald PC. Keratinocyte growth factor expression in the mesenchymal cells of human amnion. J Clin Endocrinol Metab. 1997; 82:3319–23.

33. Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000; 20:173–7.

34. Lee SB, Li DQ, Tan DT, et al. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000; 20:325–34.
Go to : 
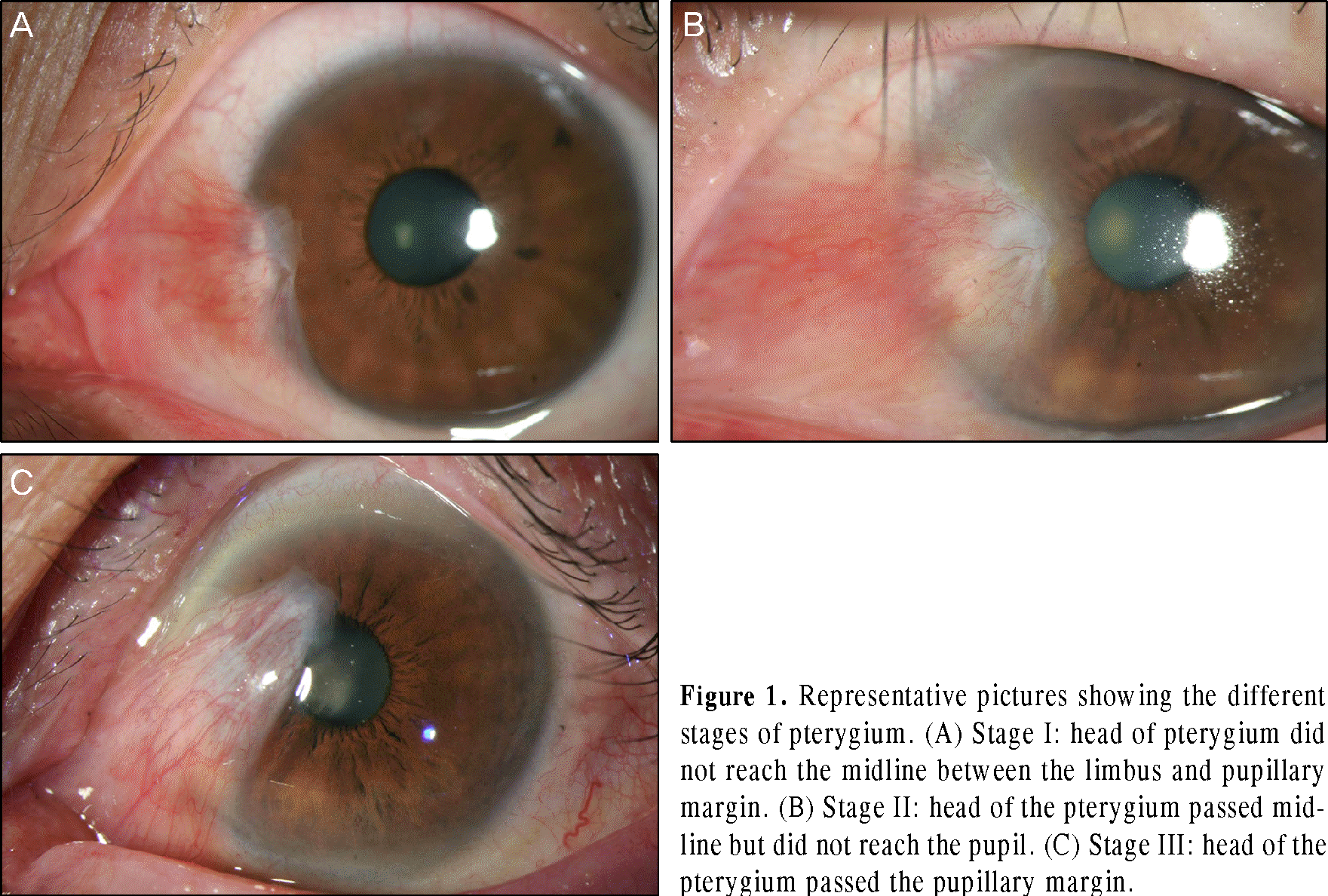 | Figure 1.Representative pictures showing the different stages of pterygium. (A) Stage I: head of pterygium did not reach the midline between the limbus and pupillary margin. (B) Stage II: head of the pterygium passed midline but did not reach the pupil. (C) Stage III: head of the pterygium passed the pupillary margin. |
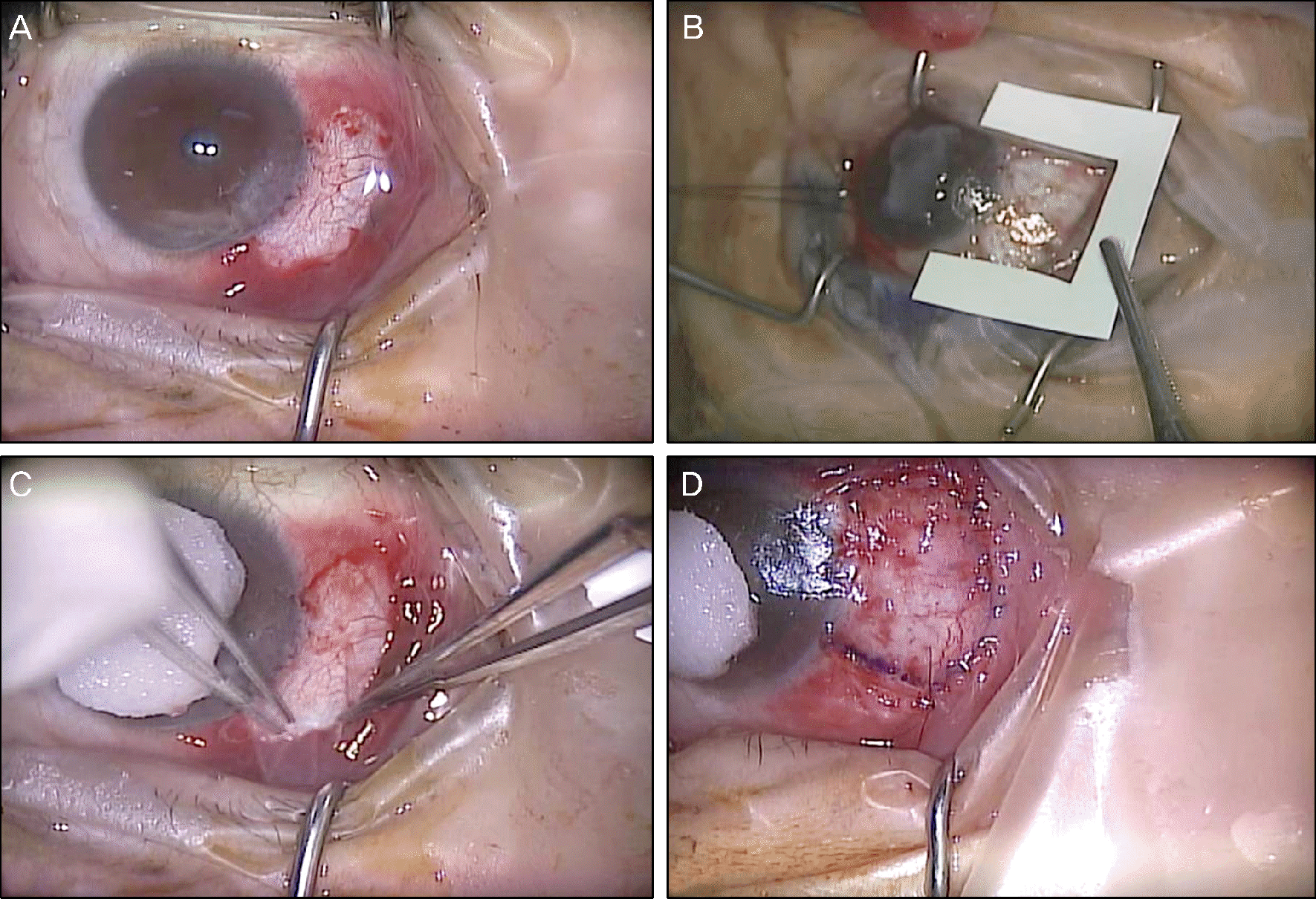 | Figure 2.Surgical steps of triple-procedure in pterygium surgery. (A) Head of pterygium, abnormal proliferative tissue, and subconjunctival fibrovascular tissue were completely removed. (B) Preparation of amniotic membrane to be inserted into the subconjunctival area. (C) Place the amniotic membrane underneath the conjunctiva along the excision margin and fixed with tissue adhesive. (D) Limbal conjunctival autograft with the use of tissue adhesive and 10-0 nylon suture. |
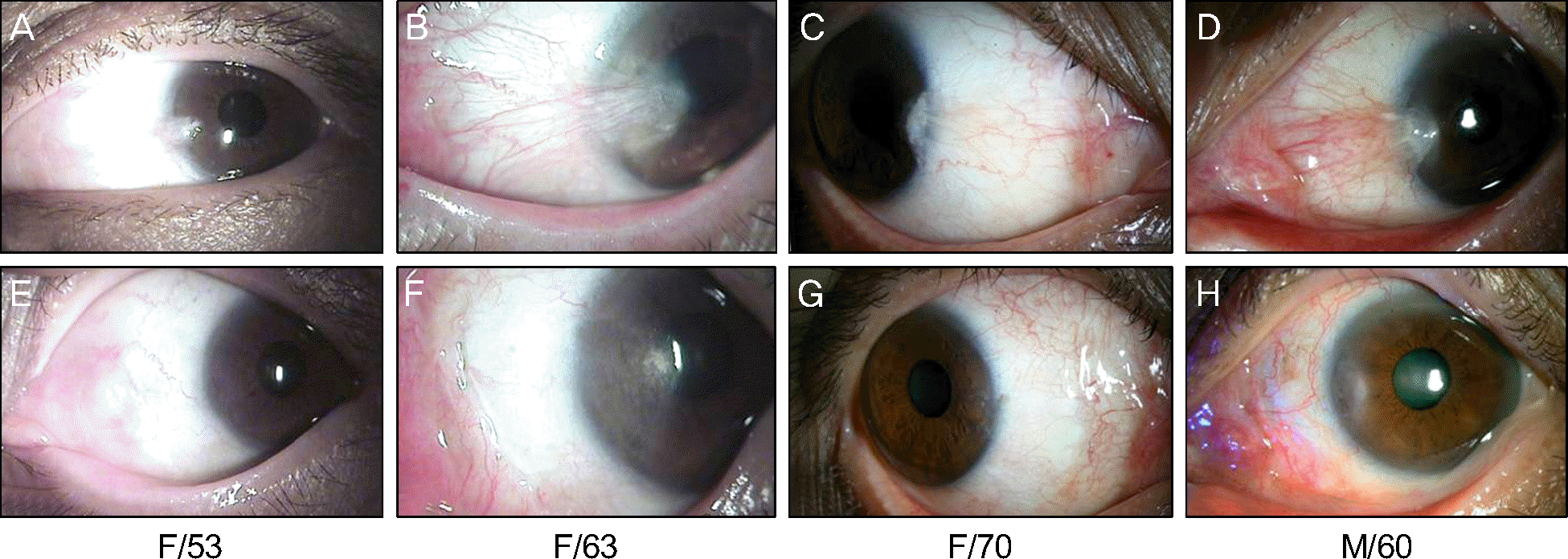 | Figure 3.Preoperative (A, B, C, D) and 6 months postoperative (E, F, G, H) anterior segment photographs of patients who had tri-ple-procedure for pterygium. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download