Abstract
Purpose
To report the corneal superficial punctate keratoepitheliopathy in 2 patients taking the epidermal growth factor re-ceptor (EGFR) inhibitors, erlotinib and lapatinib, respectively.
Case summary
Case 1, who received erlotinib, showed trichomegaly without touching the cornea and diffuse punctate keratoepitheliopathy. Corneal epitheliopathy and the corresponding symptoms resolved after discontinuation of the drug then recurred with reapplication. Case 2 presented diffuse corneal punctate epithelial erosions that developed without any cilia involvement after the patient was administered lapatinib. The visual acuity of both patients was not severely dimin-ished and keratoepitheliopathy was mostly resolved with the treatment of preservative-free artificial tears and autologous serum eye drops.
Go to : 
References
1. Modjtahedi H, Essapen S.Epidermal growth factor receptor in-hibitors in cancer treatment: advances, challenges and opportunities. Anticancer Drugs. 2009; 20:851–5.

2. Flynn JF, Wong C, Wu JM.Anti-EGFR therapy: mechanism and advances in clinical efficacy in breast cancer. J Oncol. 2009; 2009:526963.

3. Bouché O, Brixi-Benmansour H, Bertin A. . Trichomegaly of the eyelashes following treatment with cetuximab. Ann Oncol. 2005; 16:1711–2.

4. Papadopoulos R, Chasapi V, Bachariou A.Trichomegaly induced by erlotinib. Orbit. 2008; 27:329–30.

5. Foerster CG, Cursiefen C, Kruse FE.Persisting corneal erosion un-der cetuximab (Erbitux) treatment (epidermal growth factor re-ceptor antibody). Cornea. 2008; 27:612–4.

6. Johnson KS, Levin F, Chu DS.Persistent corneal epithelial defect associated with erlotinib treatment. Cornea. 2009; 28:706–7.

7. Tullo AB, Esmaeli B, Murray PI. . Ocular findings in patients with solid tumours treated with the epidermal growth factor re-ceptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Phase I and II clinical trials. Eye (Lond). 2005; 19:729–38.

8. Methvin AB, Gausas RE.Newly recognized ocular side effects of erlotinib. Ophthal Plast Reconstr Surg. 2007; 23:63–5.

9. Garibaldi DC, Adler RA.Cicatricial ectropion associated with treatment of metastatic colorectal cancer with cetuximab. Ophthal Plast Reconstr Surg. 2007; 23:62–3.

10. Zhang G, Basti S, Jampol LM.Acquired trichomegaly and sympto-matic external ocular changes in patients receiving epidermal growth factor receptor inhibitors: case reports and a review of literature. Cornea. 2007; 26:858–60.
11. Frankfort BJ, Garibaldi DC.Periocular cutaneous toxicity and cic-atricial ectropion: a potential class effect of antineoplastic agents that inhibit EGFR signaling. Ophthal Plast Reconstr Surg. 2007; 23:496–7.

12. Nakamura Y, Sotozono C, Kinoshita S.The epidermal growth fac-tor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001; 72:511–7.

13. Lyu J, Lee KS, Joo CK.Transactivation of EGFR mediates in-sulin-stimulated ERK1/2 activation and enhanced cell migration in human corneal epithelial cells. Mol Vis. 2006; 12:1403–10.
14. Förster CG, Cursiefen C, Kruse FE.Topical application of EGF for the therapy of persisting corneal erosion under cetuximab treat-ment. Ophthalmologe. 2008; 105:269–73.
15. Xu KP, Riggs A, Ding Y, Yu FS.Role of ErbB2 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2004; 45:4277–83.

16. Li T, Perez-Soler R.Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009; 4:107–19.

17. Ricciardi S, Tomao S, de Marinis F.Toxicity of targeted therapy in non-small-cell lung cancer management. Clin Lung Cancer. 2009; 10:28–35.

18. Peréz-Soler R, Saltz L.Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005; 23:5235–46.

19. Tsubota K, Goto E, Fujita H. . Treatment of dry eye by autolo-gous serum application in Sjögren's syndrome. Br J Ophthalmol. 1999; 83:390–5.

20. Matsumoto Y, Dogru M, Goto E. . Autologous serum applica-tion in the treatment of neurotrophic keratopathy. Ophthalmology. 2004; 111:1115–20.
Go to : 
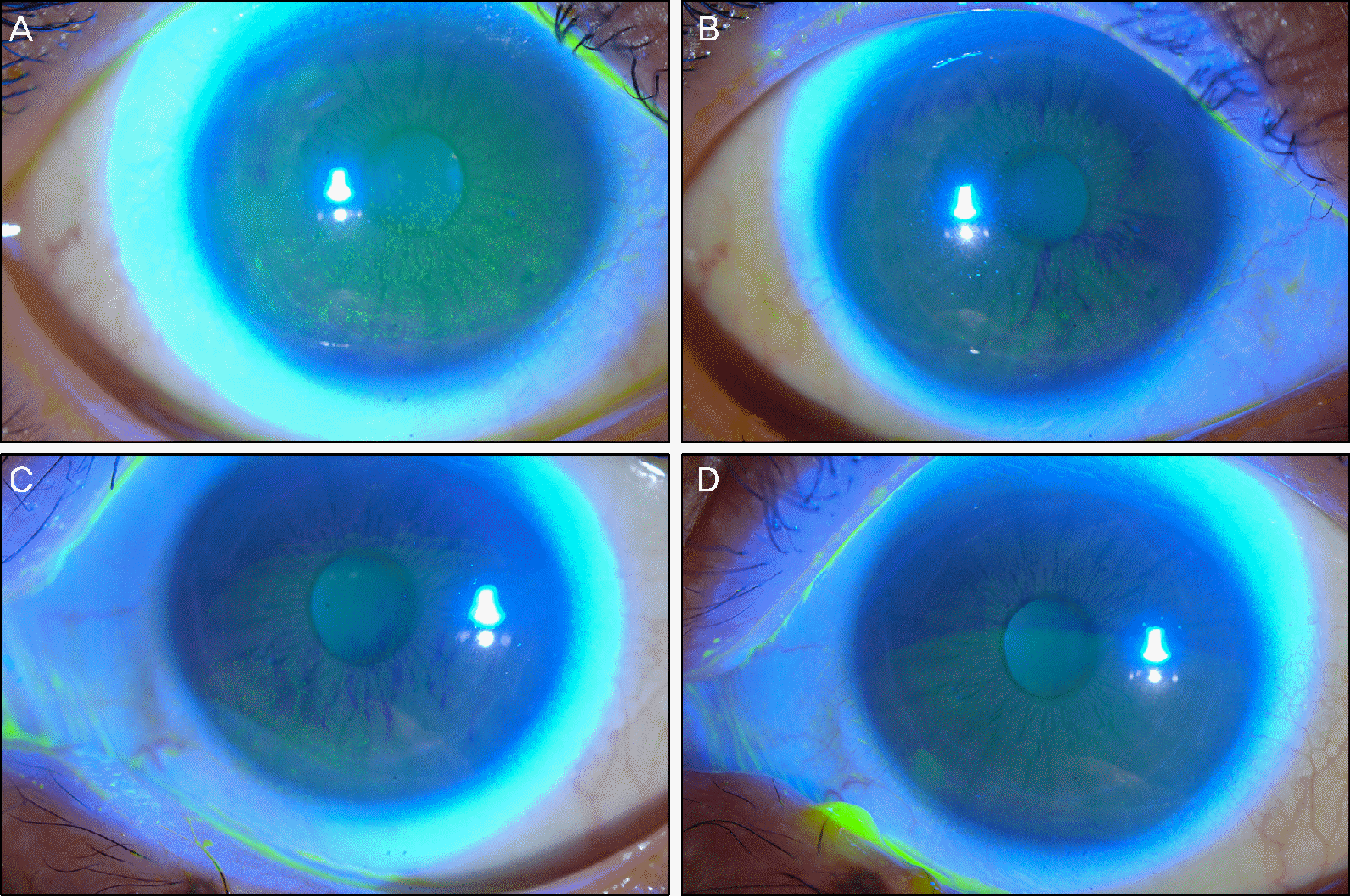 | Figure 1.Case 1 using erlotinib showed punctate epithelial erosions on the inferior halves of the cornea in both eyes at initial examinations (A: right eye, C: left eye). With preservative-free artificial tears and autologous serum eye drops, erosions nearly all disappeared (B: right eye, D: left eye). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download