Abstract
Purpose
To evaluate the effect of olopatadine and ketotifen to stabilize mast cells using human umbilical cord blood-de-rived mast cells (hCBMCs).
Methods
Using cultured hCBMCs, we divided the cells into the Ketotifen fumarate treatment group, the Olopatadine hydrochloride treatment group, the positive control group, and the negative control group. The histamine release inhibition rate was then observed.
Results
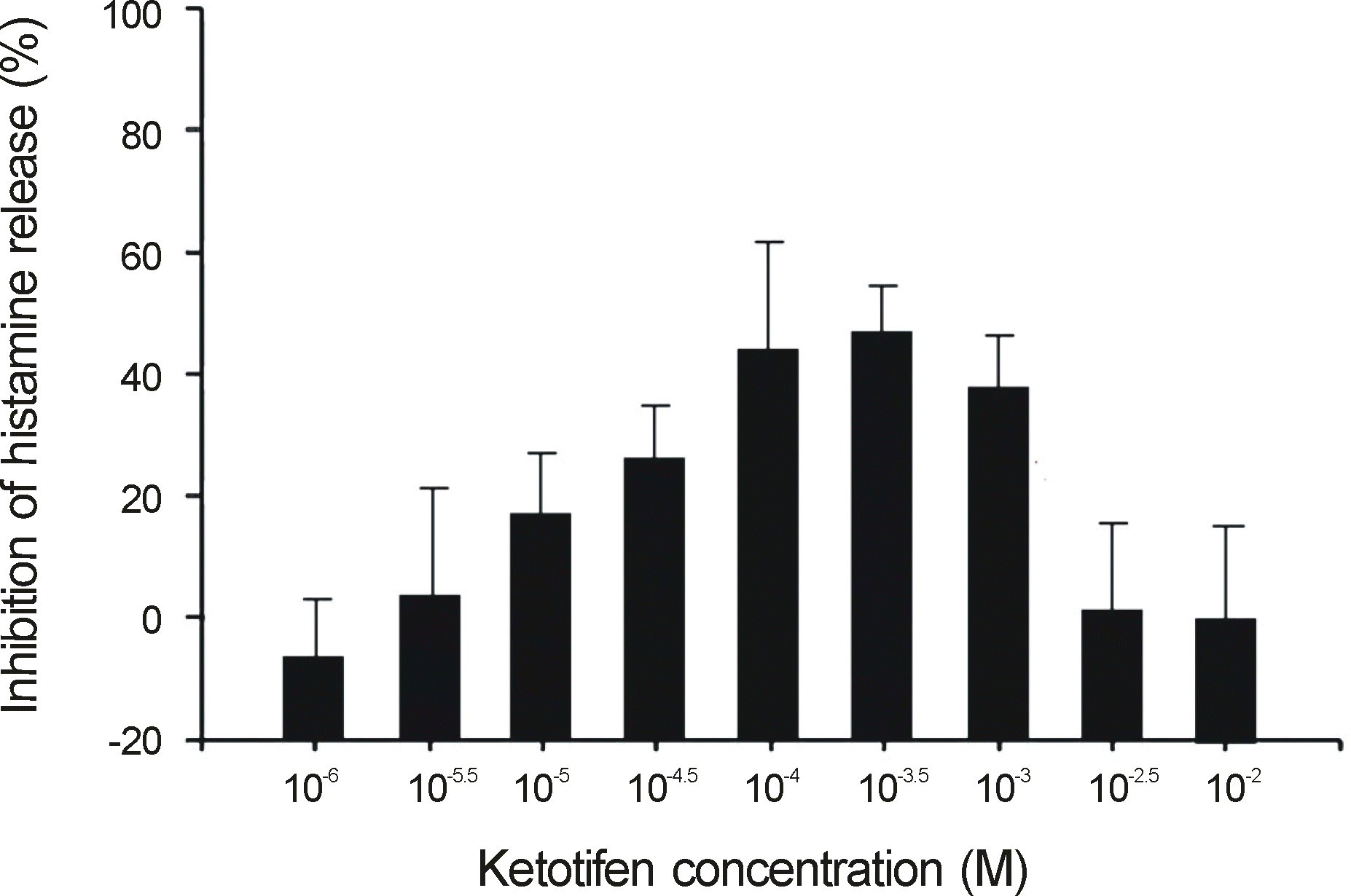
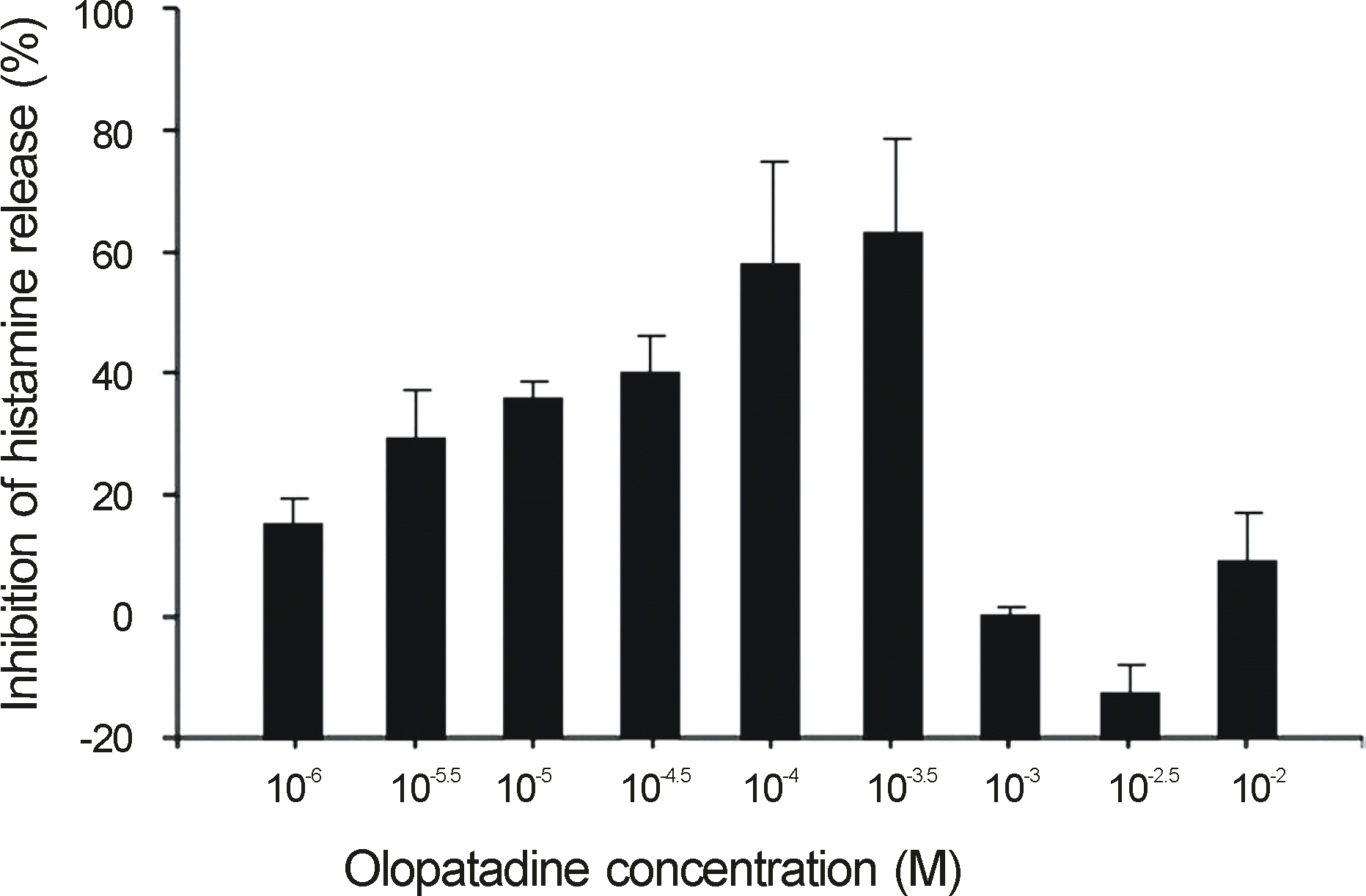
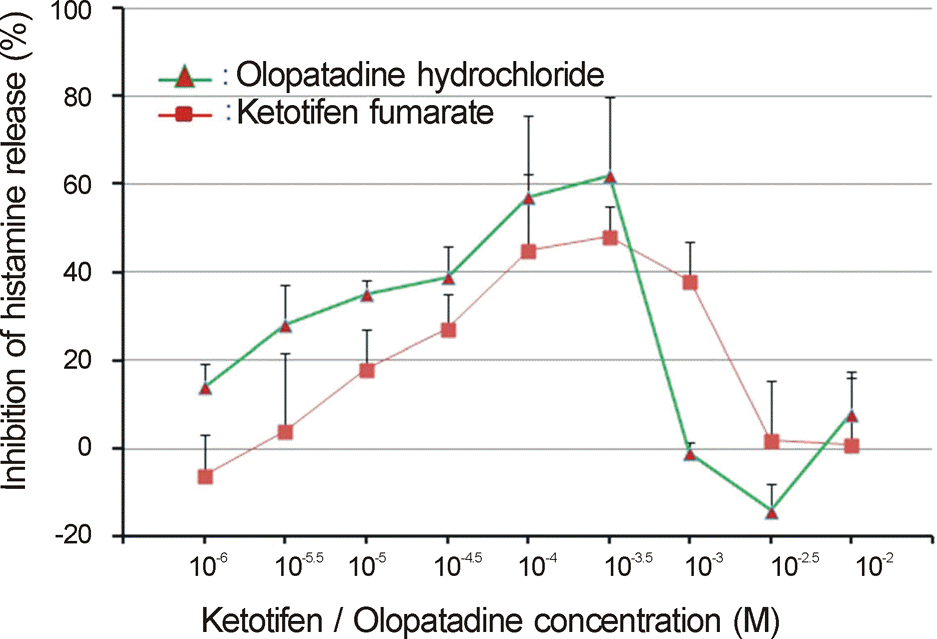
Ketotifen and olopatadine both showed the highest inhibition rate of histamine release at a concentration of 10-3.5 M (Ketotifen, 48% and Olopatadine, 62%). The histamine release inhibition rate of olopatadine was 28% at a concentration of 10-5.5 M, but ketotifen demonstrated a low histamine release inhibition rate at the same concentration. Ketotifen and olopatadine showed no histamine release inhibition at concentrations of 10-2~10-2.5 M, and 10-6 M.
References
1. Bielory L.Allergic and immunologic disorders of the eye. Part I: immunology of the eye. J Allergy Clin Immunol. 2000; 106:805–16.

2. Weeke ER.Epidemiology of hay fever and perennial allergic rhinitis. Monogr Allergy. 1987; 21:1–20.
3. Foster CS.Immunologic Disorders of the Conjunctiva. Cornea and Sclera. Principles and Practices of Ophthalmology: Section. 8:2nd ed.Philadelphia: WB Saunders Publishers;1999; 65:803–28.
4. Spangler DL, Bensch G, Berdy GJ.Evaluation of the efficacy of olopatadine hydrochloride 0.1% ophthalmic solution and azelas-tine hydrochloride 0.05% ophthalmic solution in the conjunctival allergen challenge model. Clin Ther. 2001; 23:1272–80.
5. Kidd M, McKenzie SH, Steven I. et al. Australian Ketotifen Study Group. Efficacy and safety of ketotifen eye drops in the treatment of seasonal allergic conjunctivitis. Br J Ophthalmol. 2003; 87:1206–11.
6. Abelson MB, Ferzola NJ, McWhirter CL, Crampton HJ.Efficacy and safety of single- and multiple-dose ketotifen fumarate 0.025% ophthalmic solution in a pediatric population. Pediatr Allergy Immunol. 2004; 15:551–7.

8. Kempuraj D, Saito H, Kaneko A. . Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999; 93:3338–46.

9. Kempuraj D, Asadi S, Zhang B. . Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010; 7:20.

10. Nakahata T, Toru H.Cytokines regulate development of human mast cells from hematopoietic progenitors. Int J Hematol. 2002; 75:350–6.

11. Yamaguchi M, Azuma H, Fujihara M. . Generation of a consid-erable number of functional mast cells with a high basal level of FcepsilonRI expression from cord blood CD34+ cells by co-cultur-ing them with bone marrow stromal cell line under serum-free conditions. Scand J Immunol. 2007; 65:581–8.
12. Yanni JM, Miller ST, Gamache DA. . Comparative effects of topical ocular anti-allergy drugs on human conjunctival mast cells. Ann Allergy Asthma Immunol. 1997; 79:541–5.

13. Abelson MB.Evaluation of olopatadine, a new ophthalmic anti-allergic agent with dual activity, using the conjunctival allergen challenge model. Ann Allergy Asthma Immunol. 1998; 81:211–8.

14. Fernández M, Wong E, Oehling A.Retrospective study of keto-tifen protective action in different allergopathies. Allergol Immunopathol (Madr). 1987; 15:369–73.
Figure 1.
(A) Histamine ELISA kit (H5085, US Biological, Swampscott, Massachusetts, USA) (B) ELISA reader (TECAN. Männedorf, Switzerland).
Figure 2.
Inhibition of histamine release from human umbilical cord blood-derived cultured mast cells (hCBMCs) (% reduction from positive control) as a function of ketotifen fumarate concentration.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download