Abstract
Purpose
To investigate the relationship between the concentration of aqueous humor cytokines and the severity of diabetic retinopathy.
Methods
Thirty-six subjects were included in the control, non-proliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR) groups, each group has 12 patients. Aqueous levels of cytokines (interleukin (IL)-1a, IL-1ß, IL-2, IL-4, IL-6, IL-8, IL-10, vascular endothelial growth factor (VEGF), monocyte chemoattractant protein (MCP)-1, interferon (IFN)-γ, tumor necrosis factor (TNF)-α) were investigated according to the severity of diabetic retinopathy.
Results
When the control group was compared with the PDR and NPDR groups, aqueous levels of IL-6 were significantly higher in PDR patients than in those of both the control and NPDR groups (p = 0.016 and p = 0.003, respectively). The aqueous levels of VEGF were significantly higher in the eyes of PDR patients than in those of the control group (p = 0.003). There were no statistically significant differences between the 3 groups with regard to other cytokines.
Go to : 
References
1. Murugeswari P, Shukla D, Rajendran A, et al. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina. 2008; 28:817–24.

2. Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond). 2002; 16:242–60.

3. Chakrabarti S, Cukiernik M, Hileeto D, et al. Role of vasoactive factors in the pathogenesis of early changes in diabetic retinopathy. Diabetes Metab Res Rev. 2000; 16:393–407.

4. Kaul K, Hodgkinson A, Tarr JM, et al. Is inflammation a common retinal-renal-nerve pathogenic link in diabetes? Curr Diabetes Rev. 2010; 6:294–303.

5. Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002; 133:70–7.

6. Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002; 20:4368–80.

7. Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009; 116:73–9.

8. Pe'er J, Shweiki D, Itin A, et al. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995; 72:638–45.
9. Aiello LP, Northrup JM, Keyt BA, et al. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995; 113:1538–44.

10. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

11. Meleth AD, Agrón E, Chan CC, et al. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005; 46:4295–301.

12. Jo N, Wu GS, Rao NA. Upregulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2003; 44:4054–60.

13. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–50.

14. Demircan N, Safran BG, Soylu M, et al. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond). 2006; 20:1366–9.

15. Funatsu H, Yamashita H, Shimizu E, et al. Relationship between vascular endothelial growth factor and interleukin-6 in diabetic retinopathy. Retina. 2001; 21:469–77.

16. Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005; 243:3–8.

17. Elner SG, Elner VM, Jaffe GJ, et al. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995; 14:1045–53.

18. Elner SG, Strieter R, Bian ZM, et al. Interferon-induced protein 10 and interleukin 8. C-X-C chemokines present in proliferative diabetic retinopathy. Arch Ophthalmol. 1998; 116:1597–601.
19. Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010; 28:321–42.

20. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012; 11:633–52.

21. Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004; 88:1343–7.
22. Johnsen-Soriano S, Sancho-Tello M, Arnal E, et al. IL-2 and IFN-gamma in the retina of diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2010; 248:985–90.

23. Xie M, Hu A, Luo Y, et al. Interleukin-4 and melatonin ameliorate high glucose and interleukin-1β stimulated inflammatory reaction in human retinal endothelial cells and retinal pigment epithelial cells. Mol Vis. 2014; 20:921–8.
24. Cohen T, Nahari D, Cerem LW, et al. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996; 271:736–41.

25. Gustavsson C, Agardh CD, Agardh E. Profile of intraocular tumour necrosis factor-α and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol. 2013; 91:445–52.

26. Petrovic MG, Korosec P, Kosnik M, Hawlina M. Vitreous levels of interleukin-8 in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2007; 143:175–6.
27. de Waal Malefyt R, Abrams J, Bennett B, et al. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991; 174:1209–20.

28. de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995; 27:537–41.

29. Huang S, Ullrich SE, Bar-Eli M. Regulation of tumor growth and metastasis by interleukin-10: the melanoma experience. J Interferon Cytokine Res. 1999; 19:697–703.

30. Stearns ME, Garcia FU, Fudge K, et al. Role of interleukin 10 and transforming growth factor beta1 in the angiogenesis and metastasis of human prostate primary tumor lines from orthotopic implants in severe combined immunodeficiency mice. Clin Cancer Res. 1999; 5:711–20.
31. Silvestre JS, Mallat Z, Duriez M, et al. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000; 87:448–52.

32. Hernández C, Segura RM, Fonollosa A, et al. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005; 22:719–22.

33. Selim KM, Sahan D, Muhittin T, et al. Increased levels of vascular endothelial growth factor in the aqueous humor of patients with diabetic retinopathy. Indian J Ophthalmol. 2010; 58:375–9.

34. Camussi G, Albano E, Tetta C, Bussolino F. The molecular action of tumor necrosis factor-alpha. Eur J Biochem. 1991; 202:3–14.

35. Sin BH, Kim JY, Park JY, Park SP. Analysis of intraocular cytokines according to progression of diabetic retinopathy and macular edema in diabetic patients. J Korean Ophthalmol Soc. 2013; 54:618–26.

36. Hoekzema R, Murray PI, van Haren MA, et al. Analysis of interleukin-6 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1991; 32:88–95.
Go to : 
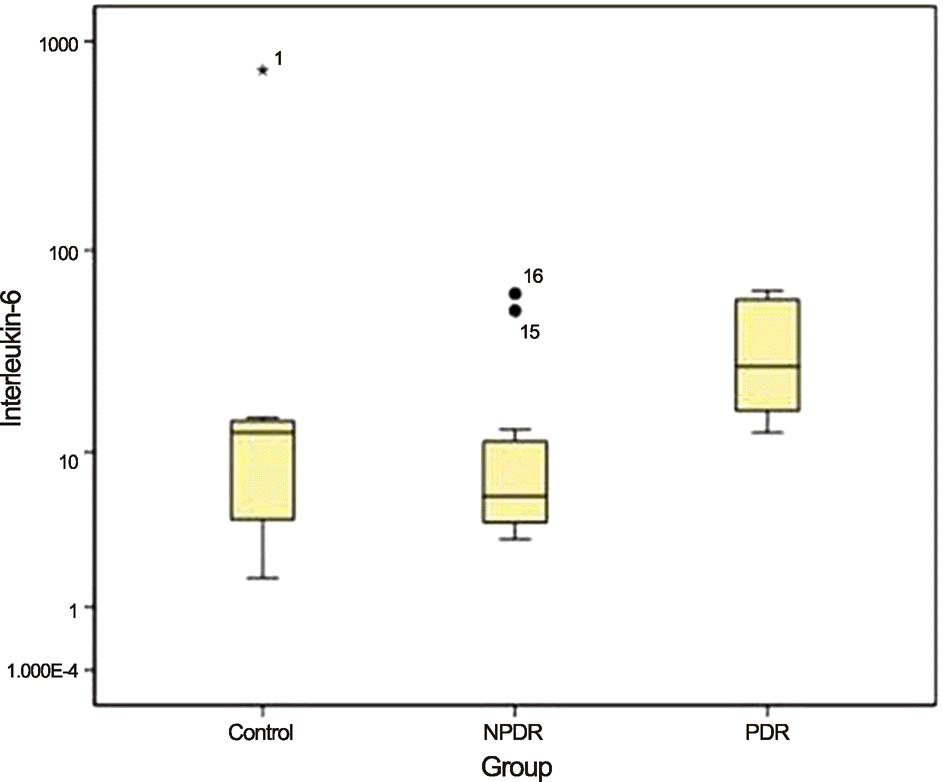 | Figure 1.Median aqueous levels of Interleukin-6 (IL-6) in each of the 3 groups are compared using Kruskal-wallis test and Dunn procedure. The levels of IL-6 in PDR group are significantly higher than the control and NPDR group (p-value = 0.016, 0.003). NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy. |
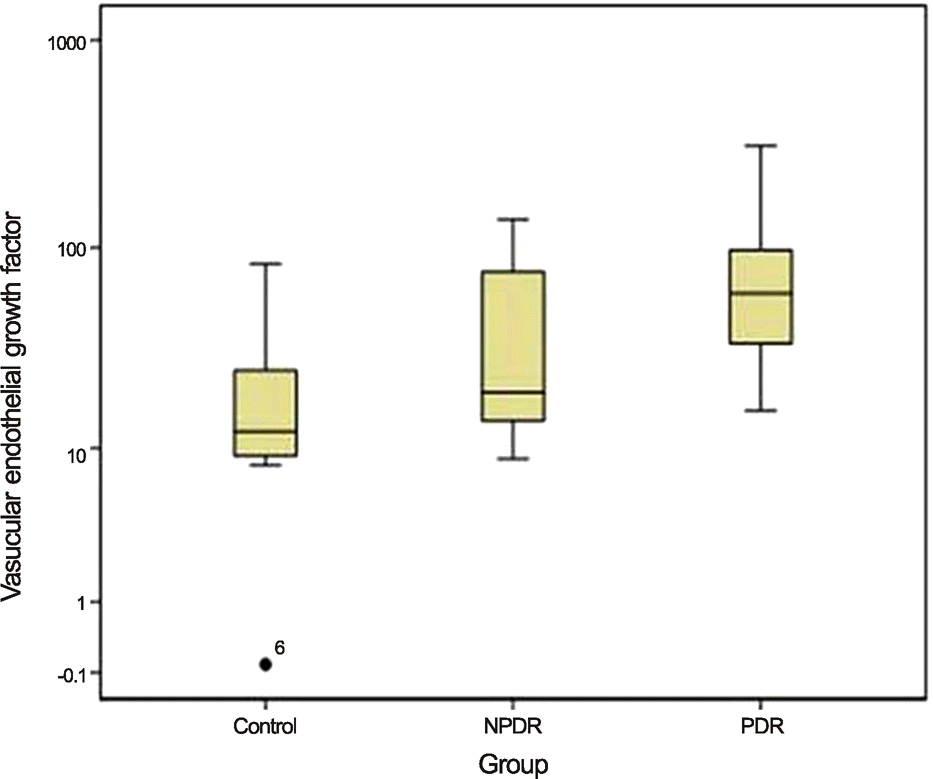 | Figure 2.Median aqueous levels of vascular endothelial growth factor (VEGF) in each of the 3 groups are compared using Kruskal-wallis test and Dunn procedure. The levels of VEGF in PDR group are significantly higher than the control group (p-value = 0.003). NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy. |
Table 1.
Subject and subgroup demographics
| Control | NPDR | PDR | Total | p-value | |
|---|---|---|---|---|---|
| Number of subjects | 12 | 12 | 12 | 36 | − |
| Mean age (years) | 50.3 ± 4.6 | 55.4 ± 9.6 | 49.2 ± 5.8 | 57.2 ± 12.6 | 0.51* |
| Gender distribution (M:F) | 5:7 | 8:4 | 7:5 | 20:16 | 0.45† |
| OD:OS | 7:5 | 6:6 | 5:7 | 18:18 | 0.72† |
Table 2.
Comparison of cytokines in the 3 groups
|
Mean ± standard deviation (pg/mL) |
Median (interquatile range) (pg/mL) |
p-value* | |||||
|---|---|---|---|---|---|---|---|
| Control | NPDR | PDR | Control | NPDR | PDR | ||
| IL-1α | 0.46 ± 0.43 | 0.46 ± 0.41 | 0.2 ± 0.38 | 0.68 (0.00-0.73) | 0.71 (0.00-0.80) | 0.00 (0.00-0.70) | 0.413 |
| IL-1β | 0.74 ± 1.07 | 0.46 ± 1.04 | 0.96 ± 1.00 | 0.50 (0.00-1.03) | 0.00 (0.00-0.74) | 1.03 (0.00-1.69) | 0.198 |
| IL-2 | 4.36 ± 1.53 | 2.86 ± 2.56 | 2.04 ± 2.49 | 4.40 (4.20-5.23) | 4.30 (0.00-5.08) | 1.00 (0.00-4.40) | 0.157 |
| IL-4 | 3.36 ± 2.86 | 2.26 ± 1.37 | 1.29 ± 1.63 | 2.95 (2.86-3.49) | 2.95 (0.71-3.06) | 0.00 (0.00-2.87) | 0.084 |
| IL-8 | 29.65 ± 42.78 | 19.21 ± 17.72 | 19.45 ± 13.69 | 10.60 (4.25-29.68) | 13.30 (7.29-29.00) | 16.45 (7.44-26.10) | 0.787 |
| IL-10 | 0.39 ± 0.49 | 0.45 ± 0.48 | 0.36 ± 0.44 | 0.00 (0.00-0.93) | 0.41 (0.00-0.92) | 0.00 (0.00-0.84) | 0.861 |
| INF-γ | 3.91 ± 6.00 | 1.61 ± 1.15 | 2.56 ± 2.60 | 2.05 (1.59-2.48) | 1.57 (0.38-2.62) | 1.81 (1.16-3.31) | 0.47 |
| TNF-α | 0.33 ± 0.77 | 0.63 ± 0.92 | 0.37 ± 0.68 | 0.00 (0.00-0.00) | 0.00 (0.00-1.83) | 0.00 (0.00-0.94) | 0.614 |
| MCP-1 | 585.53 ± 327.98 | 455.75 ± 258.20 | 669.64 ± 371.90 | 494.00 (343.28-723.88) | 387.55 (332.10-492.83) | 544.75 (435.93-672.95) | 0.094 |
| IL-6 | 68.89 ± 207.00 | 14.66 ± 19.84 | 34.69 ± 20.23 | 12.60 (4.15-14.68) | 5.92 (4.06-12.29) | 27.30 (15.75-57.90) | 0.002 |
| VEGF | 22.56 ± 24.77 | 44.15 ± 43.93 | 96.48 ± 102.80 | 12.20 (8.74-35.71) | 19.30 (13.58-88.74) | 61.42 (33.07-98.44) | 0.005 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download