Abstract
Purpose
Methods
Results
Conclusions
References
Figure 1.

Figure 2.
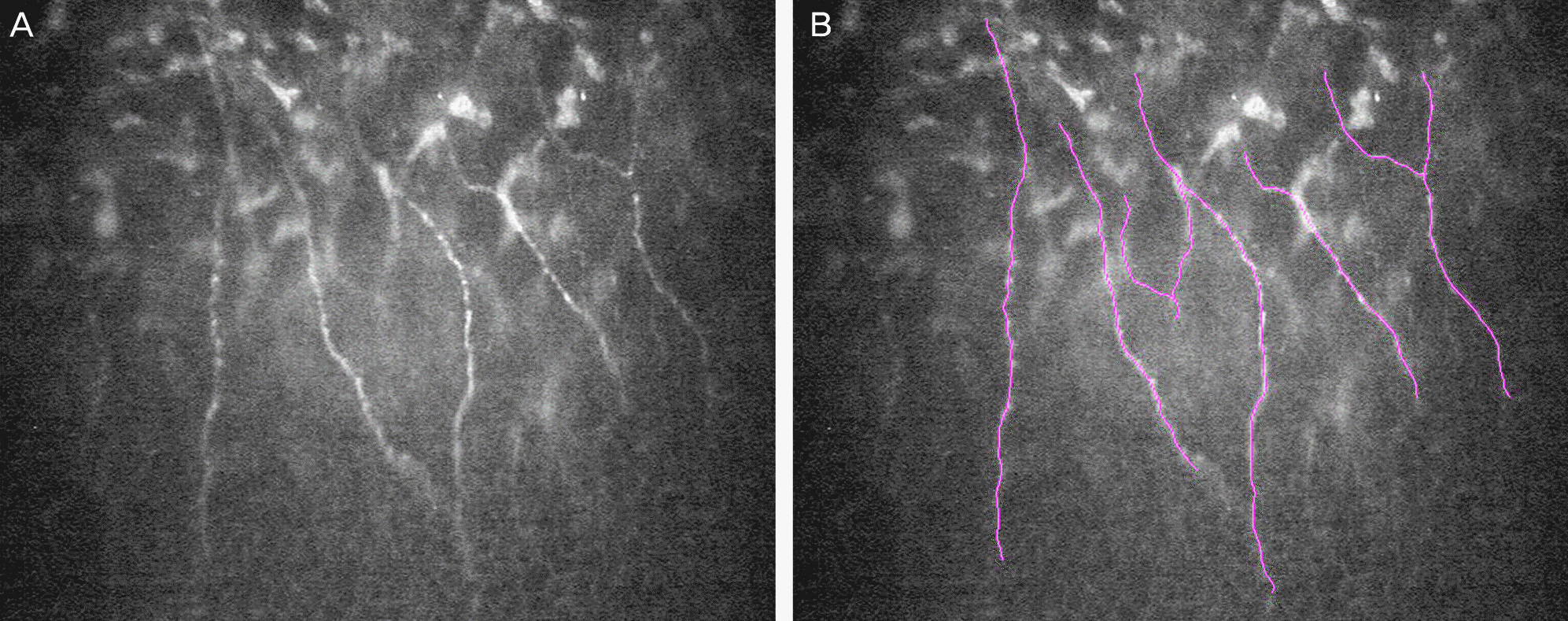
Table 1.
Values are presented as mean ± SD unless otherwise indicated; Using the Mann-Whitney U-test, no significant difference was detected in age, sex and laterality.
NK = neurotrophic keratitis; log MAR = logarithm of the minimum angle of resolution; BCVA = best-corrected visual acuity; IOP = intraocular pressure.
Table 2.
| Control | Neurosurgically induced NK | p-value | |
|---|---|---|---|
| Corneal sensitivity (cm) | 5.83 ± 0.41 | 2.67 ± 2.78 | 0.026* |
| Corneal thickness (μm) | |||
| Descemet's membrane & endothelium | 36.90 ± 15.60 | 33.45 ± 13.14 | 0.589† |
| Stroma | 408.70 ± 43.77 | 388.27 ± 110.08 | 0.579† |
| Bowman's layer | 20.10 ± 12.90 | 8.64 ± 6.25 | 0.016† |
| Epithelium | 56.70 ± 24.39 | 49.18 ± 22.42 | 0.471† |
| Total | 522.40 ± 71.05 | 479.55 ± 122.79 | 0.347† |
| Portion of cornea (%) | |||
| Descemet's membrane & endothelium | 6.90 ± 2.52 | 7.43 ± 3.48 | 0.697† |
| Stroma | 78.67 ± 5.54 | 80.45 ± 6.88 | 0.523† |
| Bowman's layer | 3.79 ± 2.37 | 1.83 ± 1.14 | 0.024† |
| Epithelium | 10.64 ± 3.74 | 10.29 ± 4.05 | 0.839† |
| Cell count (cells/mm2) | |||
| Endothelium | 2834.40 ± 265.54 | 2636.18 ± 545.97 | 0.311† |
| Stroma | |||
| Posterior keratocyte | 462.42 ± 115.51 | 450.01 ± 94.19 | 0.790† |
| Anterior keratocyte | 517.22 ± 145.57 | 532.83 ± 201.29 | 0.842† |
| Nerve analysis | |||
| Nerve fiber length (μm/mm2) | 8677.10 ± 5090.98 | 4756.59 ± 1640.91 | 0.042† |
| Number of fibers | 5.00 ± 2.11 | 4.00 ± 2.29 | 0.336† |
| Number of beadings | 2.20 ± 2.39 | 1.00 ± 1.00 | 0.172† |
| Branching pattern | 1.10 ± 1.10 | 0.78 ± 0.67 | 0.457† |
| Nerve tortuosity | 1.40 ± 1.08 | 1.00 ± 0.87 | 0.388† |
Values are presented as mean ± SD; Using the Mann-Whitney U-test, corneal sensitivity demonstrated significant differences between control and neurosurgically induced neurotrophic keratitis group; Using the independent samples t-test, the thickness and portion of Bowman's layer, total nerve fiber length demonstrated significant differences between control and neurosurgically induced neurotrophic keratitis group.
NK = neurotrophic keratitis.
Table 3.
| Corneal sensitivity | Number of fibers | Number of beading | Branching pattern | Nerve tortuosity | ||
|---|---|---|---|---|---|---|
| Nerve fiber length (μm/mm2) | Correlation coefficient | 0.268 | 0.671 | 0.663 | 0.559 | 0.489 |
| p-value | 0.354 | 0.002* | 0.002* | 0.013* | 0.034* |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download