Abstract
Purpose
To evaluate the effects of simvastatin on the catalase expression in human retinal pigment epithelium.
Methods
Retinal pigment epithelial (RPE) cells were incubated for 6 hours and 24 hours with various concentrations of simvastatin. In addition, RPE cells were incubated with 200 μM of H2O2 and various concentrations of simvastatin. After incubation, real-time polymerase chain reaction (PCR) was performed to examine the catalase messenger ribonucleic acid (mRNA) expression and a catalase assay was performed to examine the catalase activity in RPE. Intracellular reactive oxygen species (ROS) was measured using a fluorescence activated cell sorter (FACS).
Results
Simvastatin increased the amount of catalase mRNA and catalase activity at 10 μM in RPE cells. Under oxidative stress (200 μM of H2O2), 2.5 μM of simvastatin increased the catalase mRNA expression and 5 μM of simvastatin increased catalase activity in RPE cells. In addition, simvastatin reduced free radical formation but this effect was diminished in the presence of an irreversible catalase inhibitor, 3-amino-1,2,4-triazole (3-AT).
Go to : 
References
1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122:564–72.

2. Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003; 121:1621–4.
3. Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011; 129:75–80.

4. Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009; 127:533–40.
5. Yoon KC, Mun GH, Kim SD, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–33.

6. Folk JC, Stone EM. Ranibizumab therapy for neovascular age-related macular degeneration. N Engl J Med. 2010; 363:1648–55.

7. Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987; 31:291–306.

8. Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999; 5:32.
9. Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45:115–34.

11. Tate DJ Jr, Miceli MV, Newsome DA. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995; 36:1271–9.
12. Kim KJ, Kim KS, Kim NR, Chin HS. Effects of simvastatin on the expression of heme oxygenase-1 in human RPE cells. Invest Ophthalmol Vis Sci. 2012; 53:6456–64.

13. Haque R, Chun E, Howell JC, et al. MicroRNA-30b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS One. 2012; 7:e42542.

14. Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004; 61:192–208.

15. Shepherd J. Statins: is there a need for alternative or adjunctive therapy? Br Heart J. 1995; 74:13.

16. Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990; 323:1289–98.

17. Chuo JY, Wiens M, Etminan M, Maberley DA. Use of lipid-lowering agents for the prevention of age-related macular degeneration: a meta-analysis of observational studies. Ophthalmic Epidemiol. 2007; 14:367–74.

18. Pruefer D, Scalia R, Lefer AM. Simvastatin inhibits leukocyte-endothelial cell interactions and protects against inflammatory processes in normocholesterolemic rats. Arterioscler Thromb Vasc Biol. 1999; 19:2894–900.

19. Jialal I, Stein D, Balis D, et al. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001; 103:1933–5.

20. Wassmann S, Laufs U, Müller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002; 22:300–5.

21. Yamada K, Sakurai E, Itaya M, et al. Inhibition of laser-induced choroidal neovascularization by atorvastatin by downregulation of monocyte chemotactic protein-1 synthesis in mice. Invest Ophthalmol Vis Sci. 2007; 48:1839–43.

22. Dobrucki LW, Kalinowski L, Dobrucki IT, Malinski T. Statin-stimulated nitric oxide release from endothelium. Med Sci Monit. 2001; 7:622–7.
23. Qian J, Keyes KT, Long B, et al. Impact of HMG-CoA reductase inhibition on oxidant-induced injury in human retinal pigment epithelium cells. J Cell Biochem. 2011; 112:2480–9.

24. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119:1417–36.
25. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309:2005–15.
26. Peponis V, Chalkiadakis SE, Bonovas S, Sitaras NM. The controversy over the association between statins use and progression of age-related macular degeneration: a mini review. Clin Ophthalmol. 2010; 4:865–9.
27. Guymer RH, Dimitrov PN, Varsamidis M, et al. Can HMG Co-A reductase inhibitors (“statins”# slow the progression of age-related macular degeneration? The age-related maculopathy statin study #ARMSS#. Clin Interv Aging. 2008; 3:581–93.
28. Friedman E, Rigas IK, Makar M. The relationship of statin use to the development of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005; 46:199.
29. Delcourt C, Michel F, Colvez A, et al. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001; 8:237–49.

30. van Leeuwen R, Klaver CC, Vingerling JR, et al. Cholesterol and age-related macular degeneration: is there a link? Am J Ophthalmol. 2004; 137:750–2.

31. Drobek-Słowik M, Karczewicz D, Safranow K, et al. [Use of statins as a form of protection against age-related macular degeneration (AMD)]. Klin Oczna. 2008; 110:50–4.
32. Fong DS, Contreras R. Recent statin use and 1-year incidence of exudative age-related macular degeneration. Am J Ophthalmol. 2010; 149:955–8.e1.

33. Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134:411–31.

34. Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001; 73:887–96.

35. Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000; 14:835–46.

36. Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation /CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001; 286:64–70.
37. Lopez PF, Sippy BD, Lambert HM, et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996; 37:855–68.

38. Simons M. Molecular multitasking: statins lead to more arteries, less plaque. Nat Med. 2000; 6:965–6.

39. Dichtl W, Dulak J, Frick M, et al. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003; 23:58–63.

40. Frank RN, Amin RH, Puklin JE. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am J Ophthalmol. 1999; 127:694–709.
41. Olsen RH, Johnson LA, Zuloaga DG, et al. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem. 2013; Feb 5. [Epub ahead of print].

42. Gáspár T, Domoki F, Lenti L, et al. Neuroprotective effect of adenoviral catalase gene transfer in cortical neuronal cultures. Brain Res. 2009; 1270:1–9.

43. Milton NG. Inhibition of catalase activity with 3-amino-triazole enhances the cytotoxicity of the Alzheimer's amyloid-beta peptide. Neurotoxicology. 2001; 22:767–74.
44. Piechota-Polanczyk A, Goraca A, Demyanets S, et al. Simvastatin decreases free radicals formation in the human abdominal aortic aneurysm wall via NF-κB. Eur J Vasc Endovasc Surg. 2012; 44:133–7.

45. Wassmann S, Laufs U, Müller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002; 22:300–5.

46. Broncel M, Koter-Michalak M, Chojnowska-Jezierska J. [The effect of statins on lipids peroxidation and activities of antioxidants enzymes in patients with dyslipidemia]. Przegl Lek. 2006; 63:738–42.
Go to : 
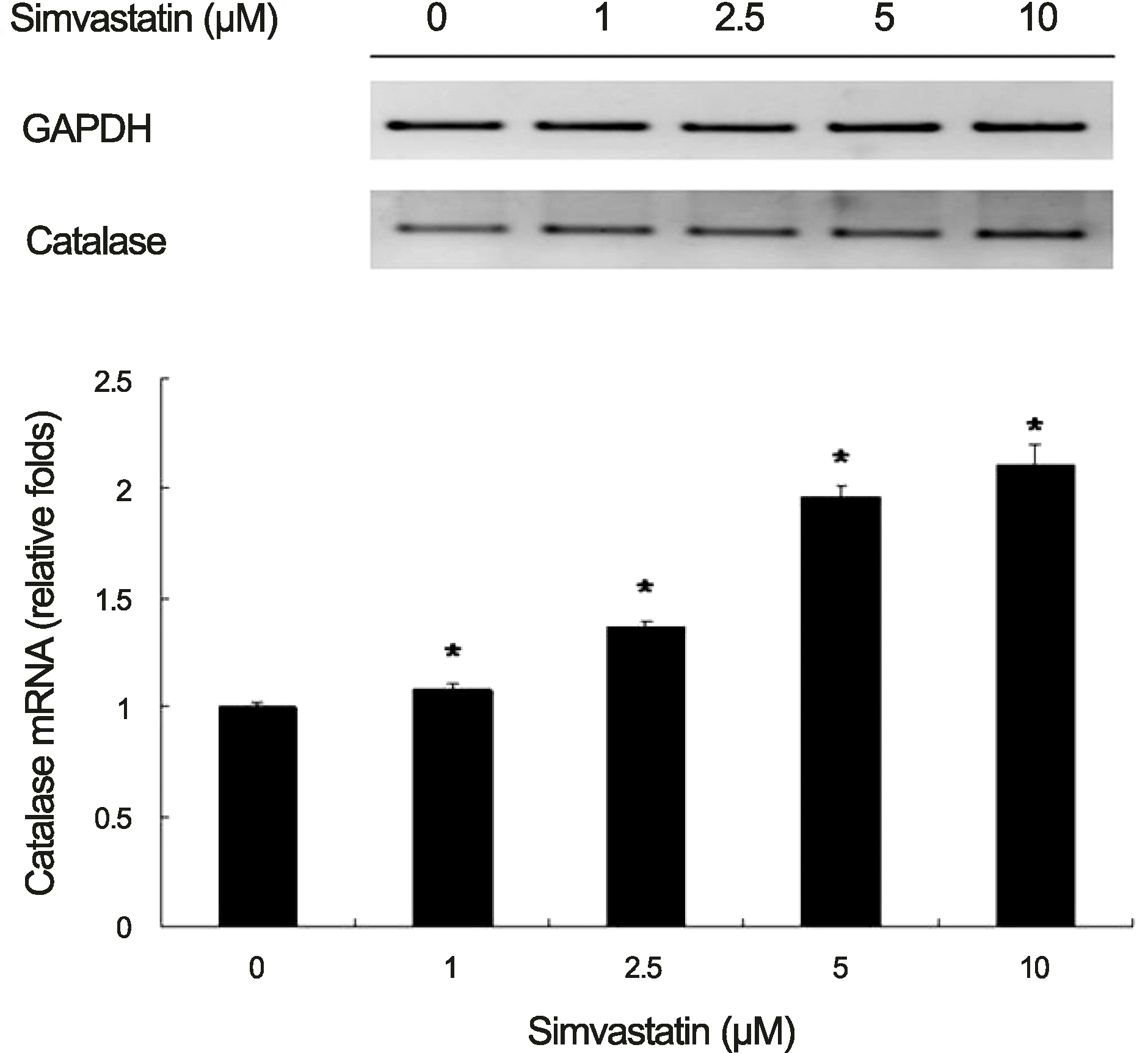 | Figure 1.Expression of catalase mRNA in human retinal pigment epithelium increased with increasing concentrations of simvastatin. Human retinal pigment epithelium was cultured in various concentrations of simvastatin for 6 hours. Expression of catalase messenger ribonucleic acid (mRNA) was calibrated using glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The experiments were performed 3 times independently and the data are shown as means ± SD. *p < 0.05 versus the control. |
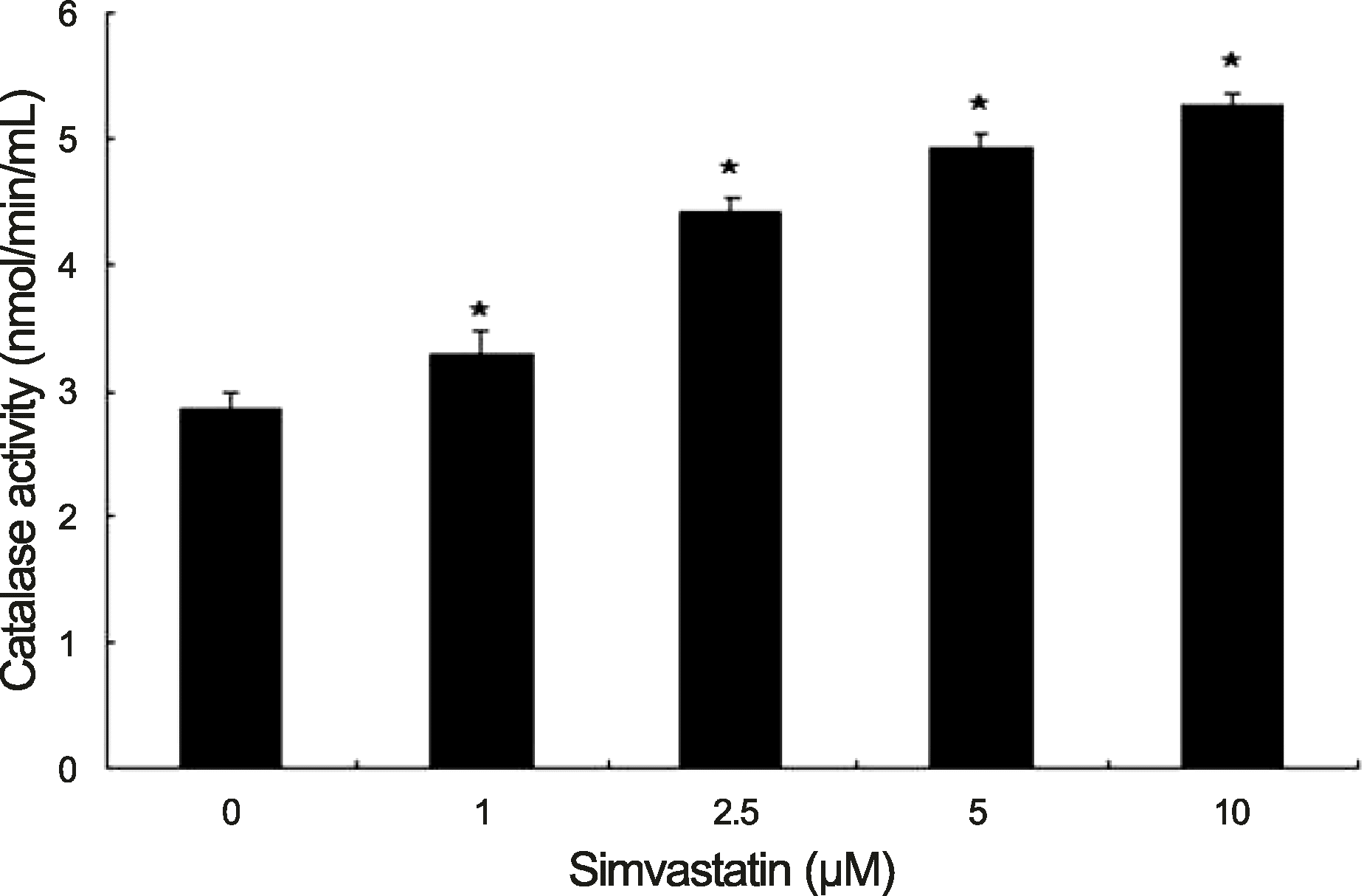 | Figure 2.Catalase activity increased with increasing concentrations of simvastatin in human retinal pigment epithelium. Human retinal pigment epithelium was cultured with various concentrations of simvastatin for 24 hours. The experiments were performed 3 times independently and the data are shown as means ± SD. *p < 0.05 versus the control. |
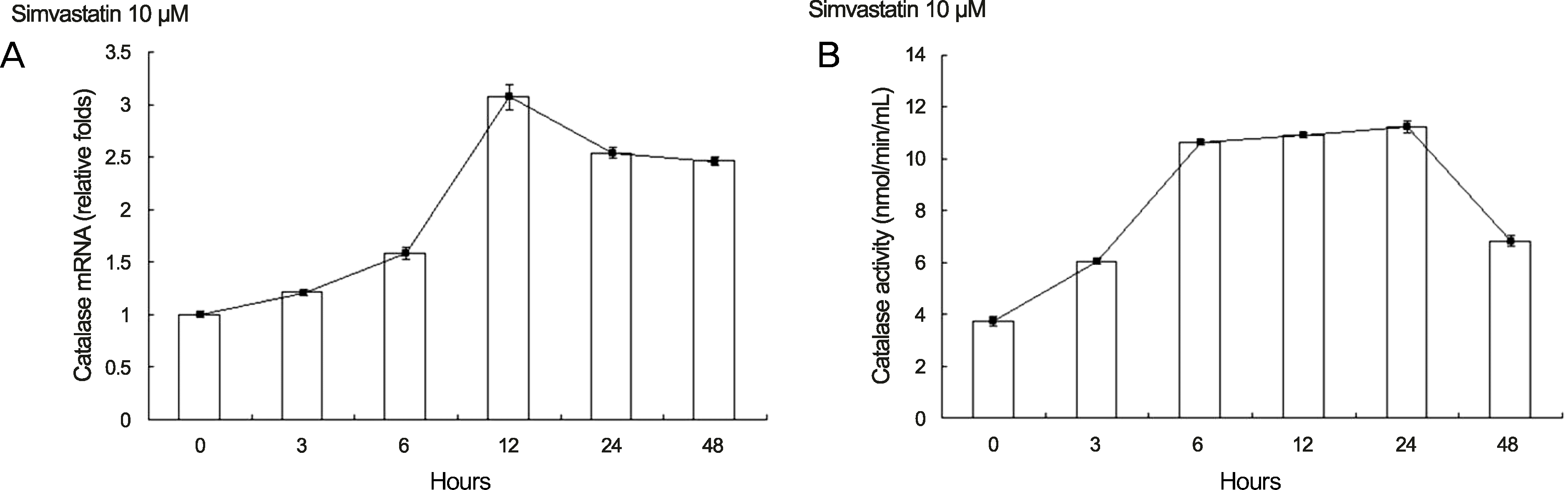 | Figure 3.Expression of catalase increased as exposure time with simvastatin increased in human retinal pigment epithelium. (A) Expression of catalase mRNA increased as exposure time with simvastatin increased in human retinal pigment epithelium. Human retinal pigment epithelium was cultured in 10 μM of simvastatin for various amounts of time. The highest level of catalase mRNA was expressed following 12 hours of culture and the effect lasted for at least 48 hours. (B) Catalase activity increased as exposure time with simvastatin increased in human retinal pigment epithelium. Human retinal pigment epithelium was cultured in 10 μM of simvastatin for various amounts of time. Catalase activity increased significantly after 6 hours. Catalase activity reached highest levels at 24 hours and the increase lasted for at least 48 hours. The experiments were performed 3 times independently and the data are shown as means ± SD. |
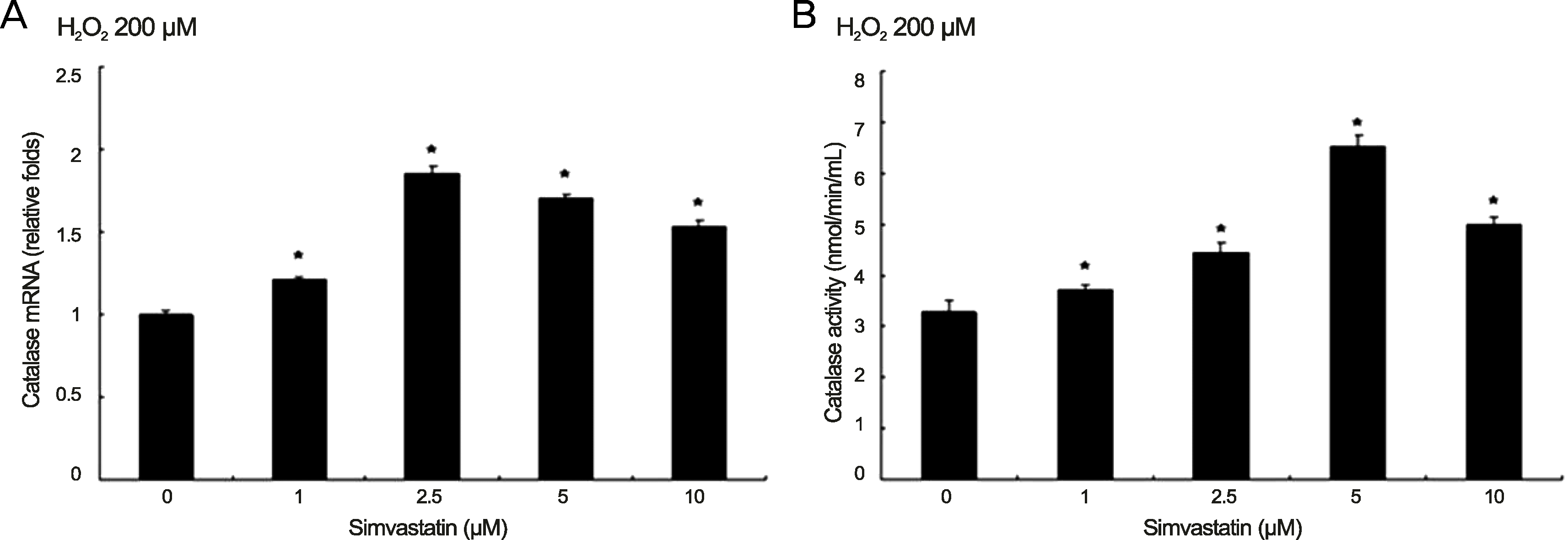 | Figure 4.Expression of catalase increased as the concentration of simvastatin increased under oxidative stress (H2O2) in human retinal pigment epithelium. (A) Expression of catalase messenger ribonucleic acid (mRNA) increased as the concentration of simvastatin increased under oxidative stress in human retinal pigment epithelium. Human retinal pigment epithelium was cultured in 200 μM of H2O2 and various concentrations of simvastatin for 6 hours. The highest level of catalase mRNA was expressed with 2.5 μM of simvastatin and the effect lasted by 10 μM of simvastatin. Expression of catalase mRNA was calibrated using glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) Human retinal pigment epithelium was cultured in 200 μM of H2O2 and various concentrations of simvastatin for 24 hours. Catalase activity reached the highest levels with 5 μM of simvastatin. The effects of catalase activity induced by simvastatin diminished with 10 μM of simvastatin. The experiments were performed 5 times independently and the data are shown as means ± SD. |
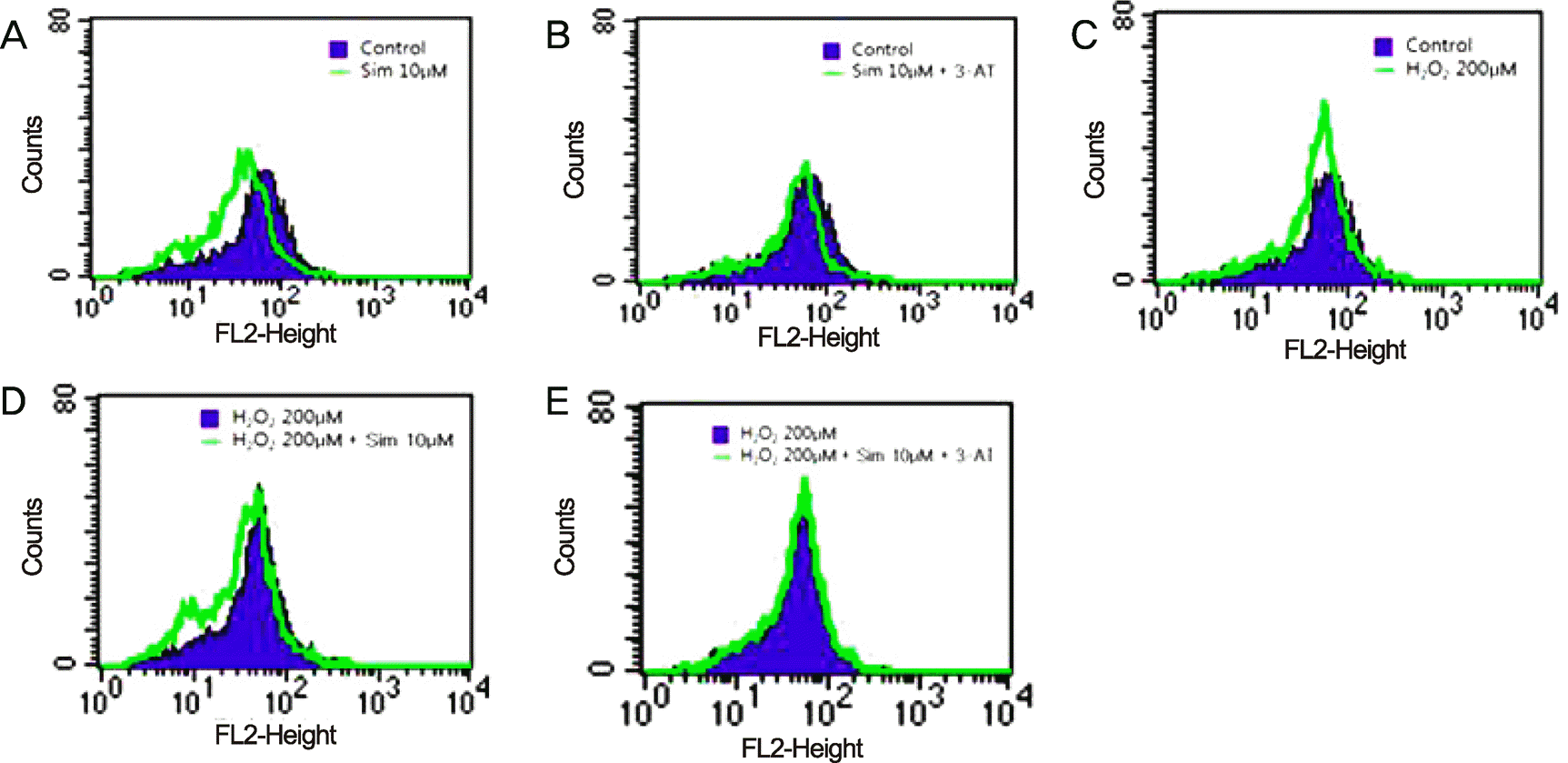 | Figure 5.The effects of simvastatin on reactive oxygen species (ROS) formation in human retinal pigment epithelium. (A) Simvastatin inhibited ROS formation in human retinal pigment epithelial cells. (B) The anti-oxidative effect of simvastatin diminished in the presence of the catalase inhibitor, 3-AT. (C) ROS formation increased following exposure to H2O2 in human retinal pigment epithelial cells. (D) Simvastatin inhibited ROS formation in hum an retinal pigment epithelial cells under oxidative stress. (E) The anti-oxidative effect of simvastatin was diminished in the presence of the catalase inhibitor, 3-AT, under oxidative stress. The data are reported as means ± SD of 3 experiments performed independently. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download