Abstract
Purpose
We present a case of acquired ocular motor apraxia accompanied with esotropia due to multiple brain infarcts.
Case summary
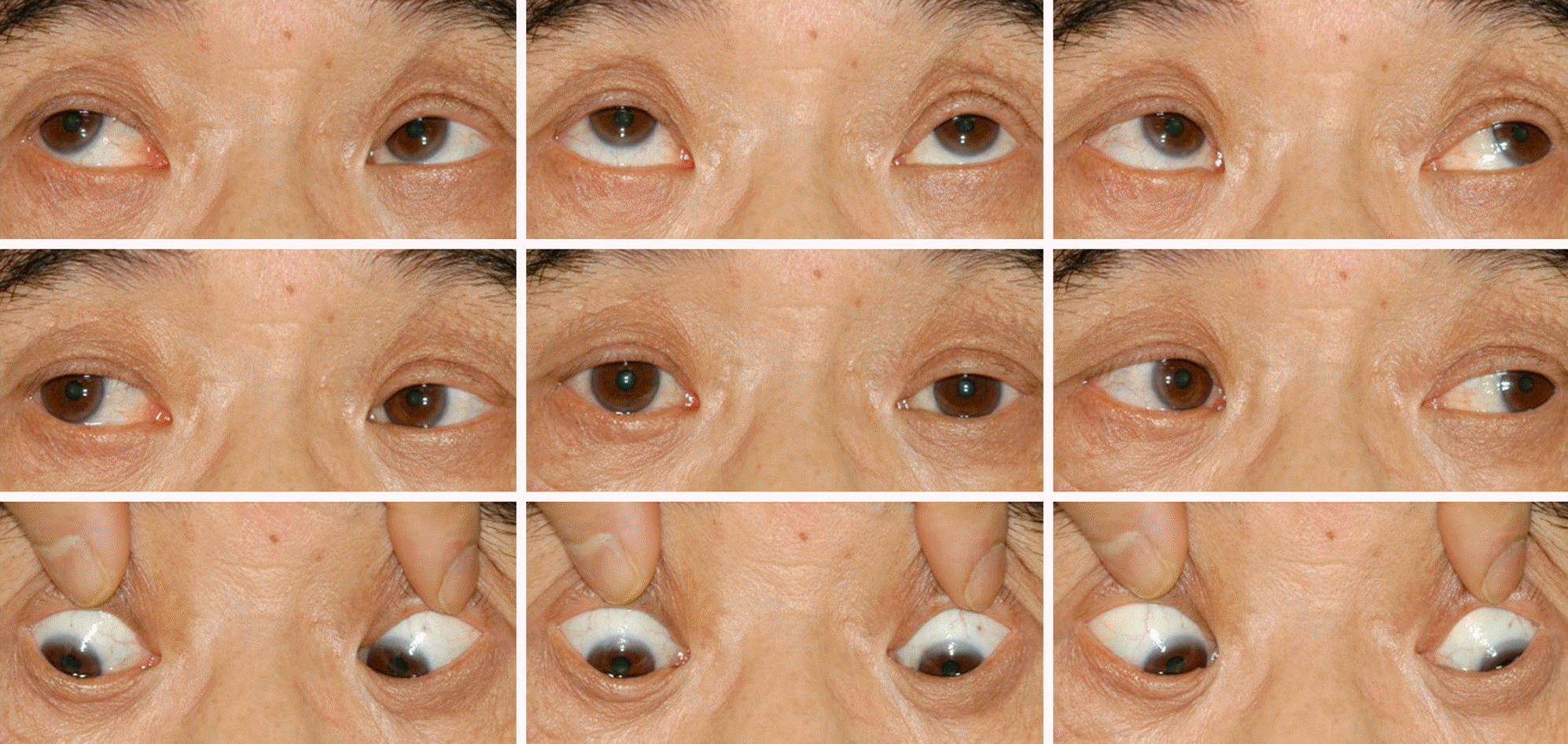
A 59-year-old male was referred for diplopia that started 9 years before presentation and continued after multiple brain infracts including right cerebellum, right occipital lobe, medulla oblongata and inferior pons. At initial examination, his best corrected visual acuity was 20/22 in the right eye and 20/25 in the left eye and he had 12 prism diopter (PD) esotropia at distance and near in primary gaze with correction. His duction and version were normal; however, his horizontal saccade was notably decreased. Two years and 8 months after presentation, the patient had 15 PD esotropia at distance and near with correction. His duction and version were normal and vertical saccadic eye movements were observed. However, horizontal saccade disappeared. The patient also exhibited a distinguishing head thrust following the order for saccadic eye movement. He was diagnosed with an acquired ocular motor apraxia accompanied with esotropia. During the follow-up period the patient underwent bilateral recession of the medial rectus. The usual diplopia and his horizontal esodeviation improved to 3 PD of esotropia at distance.
References
1. Pierrot-Deseilligny C, Gautier JC, Loron P. Acquired ocular motor apraxia due to bilateral frontoparietal infarcts. Ann Neurol. 1988; 23:199–202.
2. Cogan DG. A type of congenital ocular motor apraxia presenting jerky head movements. Am J Ophthalmol. 1953; 36:433–41.

3. Chung PW, Moon HS, Song HS, Kim YB. Ocular motor apraxia after sequential bilateral striatal infarctions. J Clin Neurol. 2006; 2:134–6.

4. Yee RD, Purvin VA. Acquired ocular motor apraxia after aortic surgery. Trans Am Ophthalmol Soc. 2007; 105:152–8.
5. Coubard OA. Saccade and vergence eye movements: a review of motor and premotor commands. Eur J Neurosci. 2013; 38:3384–97.

6. Dodge R. Five types of eye movement in the horizontal meridian plane of the field of regard. Am J Physiol. 1903; 8:307–29.

7. Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Mathematical Biosciences. 1975; 24:191–204.

8. Kondo A, Saito Y, Floricel F, et al. Congenital ocular motor apraxia: clinical and neuroradiological findings, and long-term intellectual prognosis. Brain Dev. 2007; 29:431–8.

9. Genç BO, Genç E, Açik L, et al. Acquired ocular motor apraxia from bilateral frontoparietal infarcts associated with Takayasu arteritis. J Neurol Neurosurg Psychiatry. 2004; 75:1651–2.
10. Devere TR, Lee AG, Hamill MB, et al. Acquired supranuclear ocular motor paresis following cardiovascular surgery. J Neuroophthalmol. 1997; 17:189–93.

11. Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987; 57:1033–49.

12. Le Ber I, Moreira MC, Rivaud-Péchoux S, et al. Cerebellar ataxia with oculomotor apraxia type 1: clinical and genetic studies. Brain. 2003; 126:2761–72.

13. Lee S, Kim HJ, Jeong SY, Hwang JM. Ocular abnormality of Korean patients with molecular genetically confirmed Gaucher disease. J Korean Ophthalmol Soc. 2013; 54:131–5.

Figure 1.
T2-weighted axial brain magnetic resonance imaging (MRI) demonstrated high signal intensities of the right cerebellum (arrows) (A), and medulla oblongata (arrow) (B) 9 years before presentation. Axial diffusion MRI also showed high signal intensities of pons (arrow) (C), and the right occipital lobe (arrow) (D) at the same time. One year after presentation, axial MRI showed persistent lesion of the pons obtained on T2-weighted image (arrow) (E) and that of the occipital lobe obtained on flair image (arrow) (F) without significant interval change.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download