Abstract
Purpose
To investigate the effects of commodified growth factor products used clinically on fibrovascular ingrowth into porous polyethylene orbital implants.
Methods
Porous polyethylene orbital implant sheets (Medpor®) soaked with Nepidermin (Easyef®), Trafermin (Fiblast®), and normal saline were implanted into the backs of 18 Sprague-Dawley rats. The degree of fibrovascular ingrowth as observed using a light microscope was compared 1 and 2 weeks after implantation and was calculated as a percentage of the fibrovascular ingrowth length.
Results
One week after implantation, the percentage of fibrovascular ingrowth length was 25.33 ± 5.43%, 22.56 ± 5.30%, and 21.78 ± 4.66% in the Easyef®-, Fiblast®- and normal saline-soaked groups. The degree of fibrovascularization was higher in the Easyef®-soaked group than in the other groups (p = 0.020, 0.012). Two weeks after implantation, the degree of fibrovascularization was 98.33 ± 5.00%, 100.00 ± 0.00%, and 95.89 ± 4.57%, which was significantly higher in the Easyef®-, and Fiblast®-soaked groups than in normal saline-soaked group (p = 0.019, <0.001).
Go to : 
References
1. Rubin PA, Popham JK, Bilyk JR, Shore JW. Comparison of fibrovascular ingrowth into hydroxyapatite and porous polyethylene orbital implants. Ophthal Plast Reconstr Surg. 1994; 10:96–103.

2. Romano JJ, Iliff NT, Manson PN. Use of Medpor® porous polyethylene implants in 140 patients with facial fractures. J Craniofac Surg. 1993; 4:142–7.

3. Yoon HJ, Kim SJ, Yoon SW, Yoon YS. Effects of Medpor® sheet as substitute for tarsus in eyelid reconstruction. J Korean Ophthalmol Soc. 2009; 50:1098–104.

4. Purdy EP. Oculoplastic and orbital applications of porous high-density polyethylene implants. Curr Opin Ophthalmol. 1997; 8:57–63.

5. Mawn LA, Jordan DR, Gilberg S. Scanning electron microscopic examination of porous orbital implants. Can J Ophthalmol. 1998; 33:203–9.
6. Karesh JW, Dresner SC. High-density porous polyethylene (Medpor®) as a successful anophthalmic socket implant. Ophthalmology. 1994; 101:1688–95. discussion 1695-6.

7. Nicaeus TE, Tolentino MJ, Adamis AP, Rubin PA. Sucralfate and basic fibroblast growth factor promote endothelial cell proliferation around porous alloplastic implants in vitro. Ophthal Plast Reconstr Surg. 1996; 12:235–9.

8. Soparkar CN, Wong JF, Patrinely JR, Appling D. Epidermal and fibroblast growth factors enhance fibrovascular integration of porous polyethylene implants. Ophthal Plast Reconstr Surg. 2000; 16:337–40.

9. Soe SG, Kim YI, Shin SG, Oum BS. Study on fibrovascular ingrowth into the intraocular implant, Medpor®. J Korean Ophthalmol Soc. 2002; 43:1310–22.
10. Kim KR, Jung CS, Chung SK. The effect of basic fibroblast growth factor on the vascularization of porous polyethylene orbital implant (Medpor®). J Korean Ophthalmol Soc. 2002; 43:2258–64.
11. Choi YS, Yim HB, Choi WC. The effect of vascular endothelial growth factor on vascularization in porous polyethylene orbital implant (Medpor®). J Korean Ophthalmol Soc. 2003; 44:1180–7.
12. Han SK, You HJ. Wound coverage using advanced technology in Korea. J Korean Med Assoc. 2011; 54:594–603.

13. Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg. 2006; 56:394–8.

14. Cho JH, Lew DH, Kim YS, Tark KC. Treatment of acute and chronic wound in immunodeficiency patients using Easyef® (EGF). J Korean Soc Transplant. 2006; 20:99–103.
15. Akita S, Akino K, Imaizumi T, Hirano A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen. 2008; 16:635–41.

16. Uemura T. Evaluation with trafermin for pediatric thermal burn in outpatients. NESSHO-TOKYO-. 2006; 32:291–7.
17. Ishibashi Y, Soeda S, Ohura T, et al. The clinical effects of KCB-1 on skin ulcers: a double blind trial to investigate an optimal dose. Rinsho Iyaku. 1996; 12:1809–34.
19. Woo KI, Kim JM, Chang HR, et al. The effect of growth factors and surgical procedures on fibrovascular ingrowth into anophthalmic socket implant of porous polyethylene. J Korean Ophthalmol Soc. 2000; 41:2725–31.
20. Rubin PA, Nicaeus TE, Warner MA, Remulla HD. Effect of sucralfate and basic fibroblast growth factor on fibrovascular ingrowth into hydroxyapatite and porous polyethylene alloplastic implants using a novel rabbit model. Ophthal Plast Reconstr Surg. 1997; 13:8–17.

21. Park WC, Han SK, Kim NJ, et al. Effect of basic fibroblast growth factor on fibrovascular ingrowth into porous polyethylene anophthalmic socket implants. Korean J Ophthalmol. 2005; 19:1–8.

22. Cho NC, Lee MC, Han HJ, Ahn BG. Proliferative effect of retinal glial cells by growth factors. J Korean Ophthalmol Soc. 1997; 38:1179–87.
23. Yoon HS, Rho SH, Lee SC, et al. The effects of TGF-β2 and bFGF on the proliferation of retinal pigment epithelial cells. J Korean Ophthalmol Soc. 1998; 39:1192–203.
24. Kwak NH, Cuhn MH, Yoo JS, Huh W. Neurotropic effect of basic fibroblast growth factor on laser wound. J Korean Ophthalmol Soc. 2000; 41:1666–73.
25. Hirano M, Pavlakis SG. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): current concepts. J Child Neurol. 1994; 9:4–13.
26. Kurten RC, Chowdhury P, Sanders RC Jr, et al. Coordinating epidermal growth factor-induced motility promotes efficient wound closure. Am J Physiol Cell Physiol. 2005; 288:C109–21.

27. Motomura H, Ohashi N, Harada T, et al. Aggressive conservative therapy for refractory ulcer with diabetes and/or arteriosclerosis. J Dermatol. 2006; 33:353–9.

28. Bigham WJ, Stanley P, Cahill JM Jr, et al. Fibrovascular ingrowth in porous ocular implants: the effect of material composition, porosity, growth factors, and coatings. Ophthal Plast Reconstr Surg. 1999; 15:317–25.
29. Jang JH, Chang SD. Upper eyelid reconstruction using the Medpor® sheet and median forehead flap. J Korean Ophthalmol Soc. 2009; 50:1105–10.

30. Lim JY, Jang JW, Moon SH. Lid reconstruction using the porous polyethylene (Medpor®) sheet. J Korean Ophthalmol Soc. 2003; 44:2111–6.
Go to : 
 | Figure 1.Porous polyethylene sheets soaked with Easyef® (Nepidermin, Group E), Fiblast® (Trafermin, Group F), normal saline (Group N). |
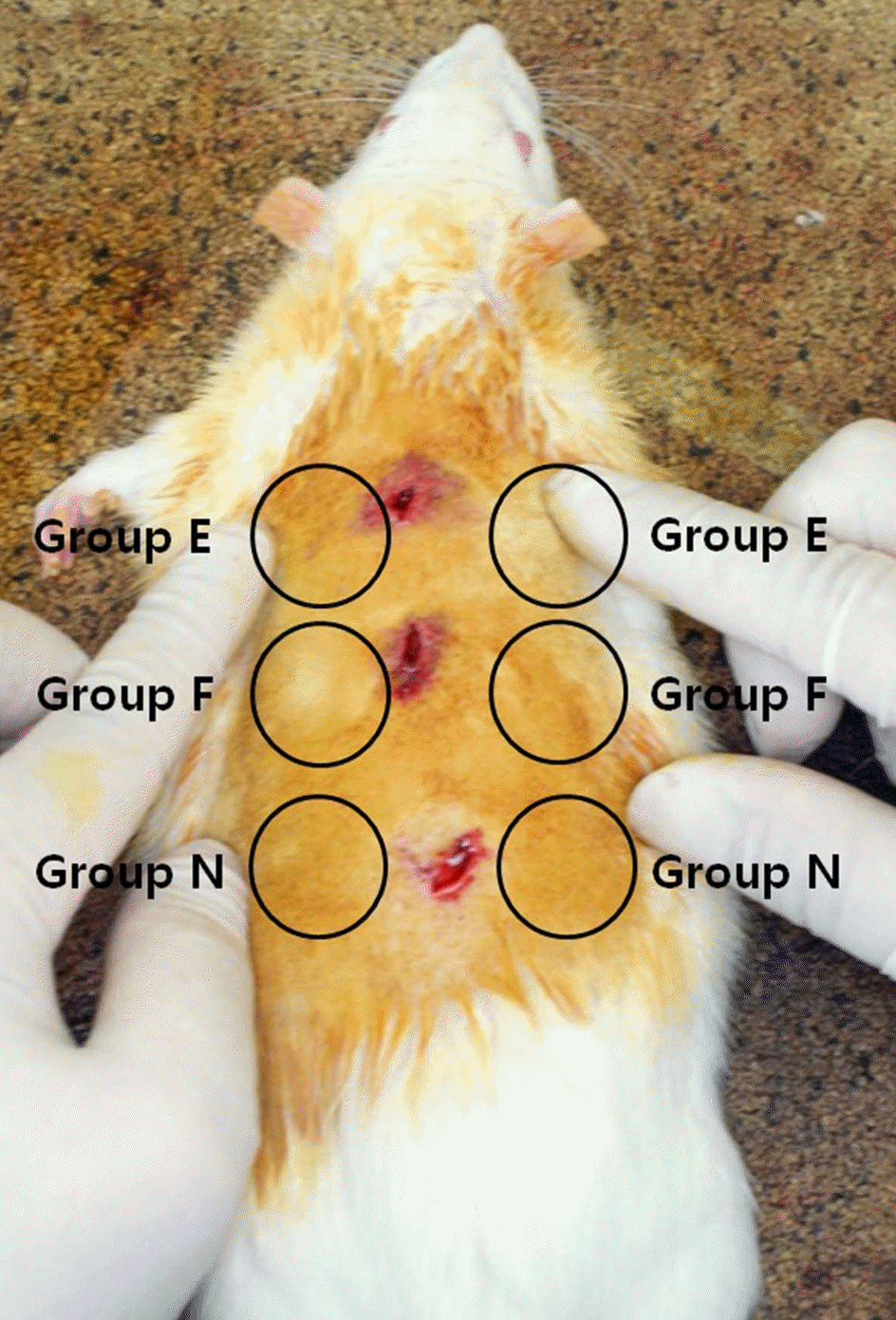 | Figure 2.Postoperative photograph of the mice. Medpor® sheets soaked with Easyef® (Nepidermin, Group E), Fiblast® (Trafermin, Group F), normal saline (Group N) were implanted into mice back. Each group was implanted on each both sides in the upper, middle, and lower part, respectively. |
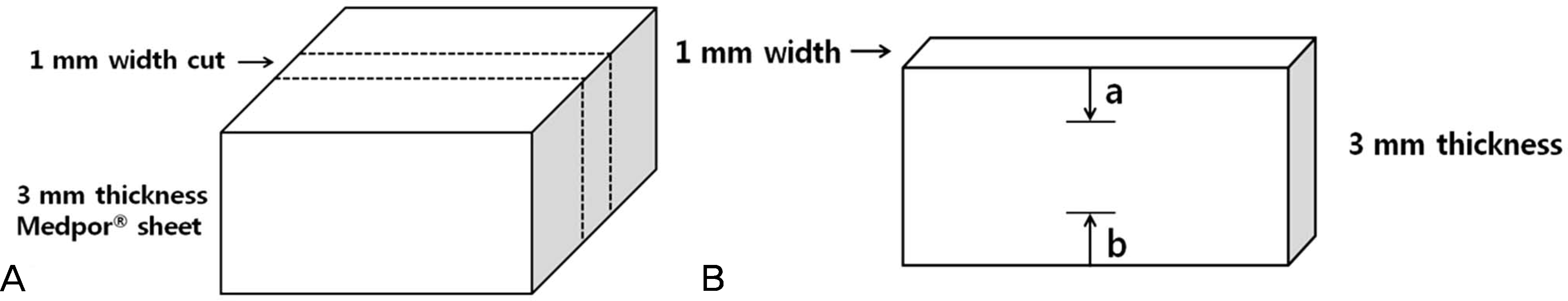 | Figure 3.Schematic diagram depicting the fibrovascular ingrowth of each sheet central plane. (A) 3 mm thickness Medpor® sheet cut into 1 mm width in the central plane of each sheet (broken line). (B) Fibrovascular ingrowth length was examined in 2 directions (a, b) and measured at 2 points. Each fibrovascular ingrowth length (%) = (a + b)/3 × 100. The degree of fibrovascularization was calculated an average of the each fibrovascular ingrowth length. |
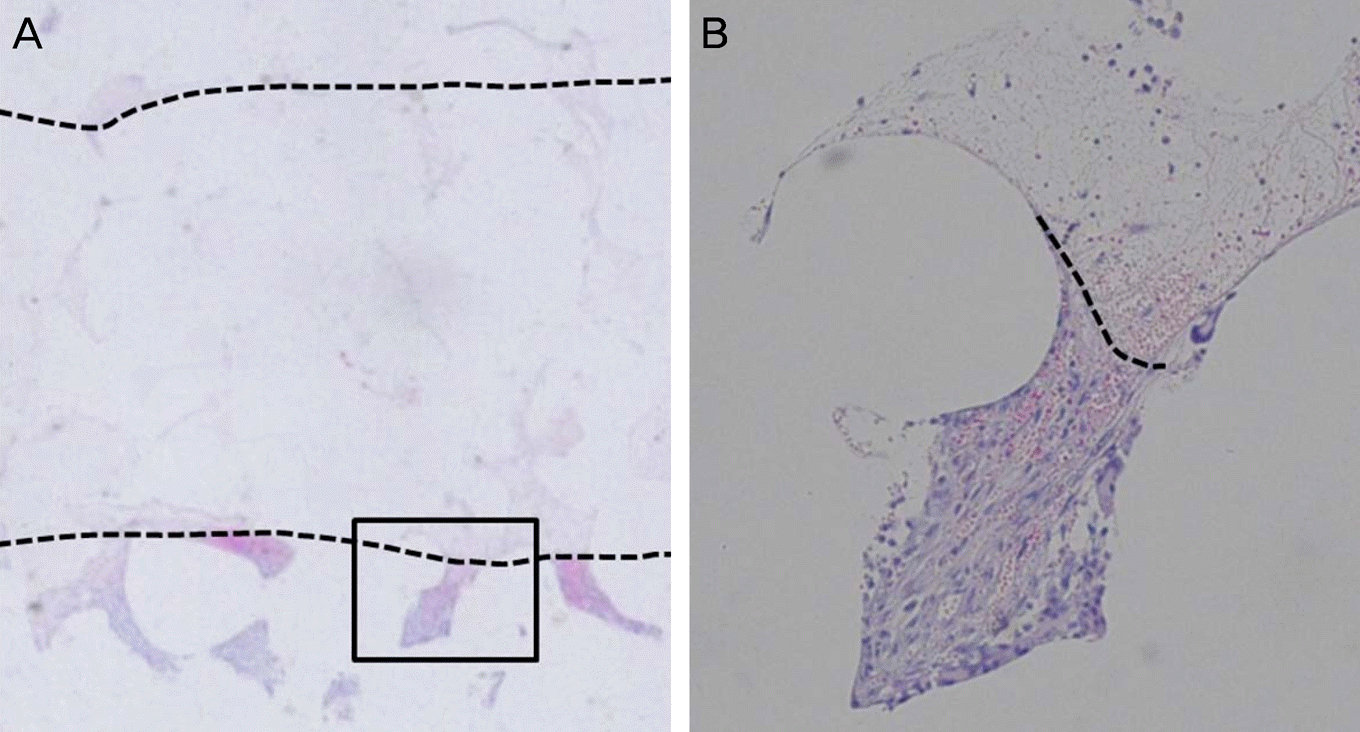 | Figure 4.Determination of fibrovascular ingrowth margin in hematoxylin & eosin staining sheet under a light microscope. This sheet is Easyef® (Nepidermin) soaked sheet at 1 week after implantation. The fibrovascular ingrowth margin was drawn with a dotted line. (A) Low magnification view (×10) of cross section of the sheet. (B) Higher magnification view (×40) of the box area in (A). A connective tissue enmeshed with spindle-shaped fibroblasts can be seen on the lower side of the picture. |
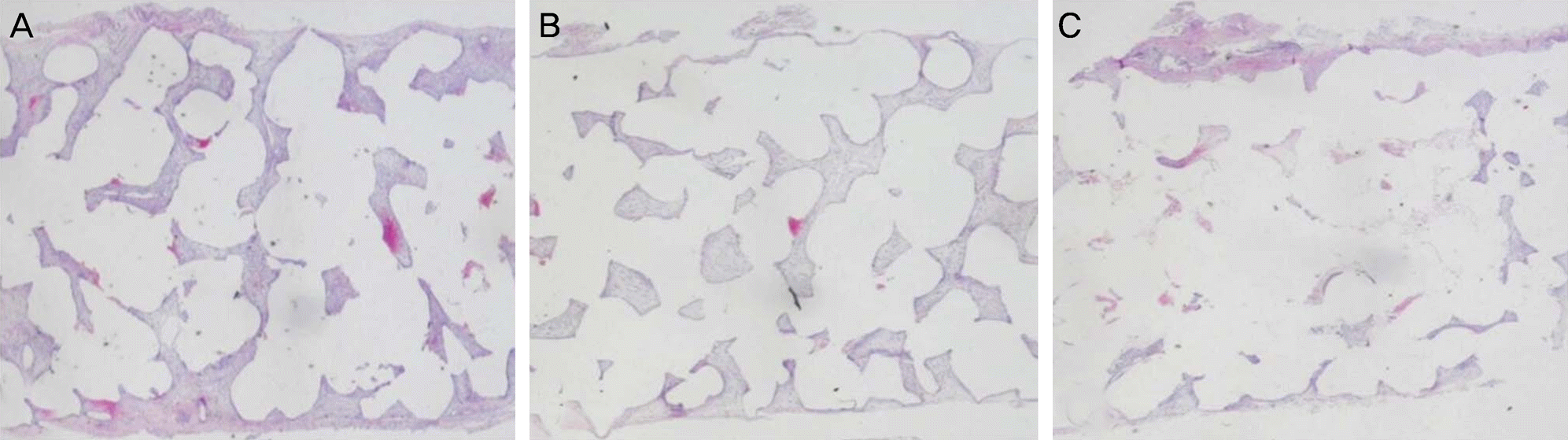 | Figure 5.Hematoxylin & eosin staining (×10) of extracted porous polyethylene sheets showing that Easyef® (Nepidermin)-, Fiblast® (Trafermin)-soaked sheets have more fibrovascularization than normal saline-soaked sheet 2 weeks after implantation. (A) Soaking of sheet in Easyef® (Nepidermin). (B) Soaking of sheet in Fiblast® (Trafermin). (C) Soaking of sheet in normal saline. |
Table 1.
Degree of fibrovascular ingrowth into the porous polyethylene sheets 1 week after implantation
| Group* | Percentage of the length of fibrovascular ingrowth† |
p-value on Mann-Whitney U-test between two groups |
|
|---|---|---|---|
|
Group |
|||
| Group E | Group F | ||
| Group E | 25.33 ± 5.43 | − | − |
| Group F | 22.56 ± 5.30 | 0.020 | − |
| Group N | 21.78 ± 4.66 | 0.012 | 0.522 |
Table 2.
Degree of fibrovascular ingrowth into the porous polyethylene sheets 2 weeks after implantation
| Group* | Percentage of the length of fibrovascular ingrowth† |
p-value on Mann-Whitney U-test between two groups |
|
|---|---|---|---|
|
Group |
|||
| Group E | Group F | ||
| Group E | 98.33 ± 5.00 | − | − |
| Group F | 100.00 ± 0.00 | 0.151 | − |
| Group N | 95.89 ± 4.57 | 0.019 | <0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download