Abstract
Purpose
To investigate the effect of low-dose systemic cyclosporine A (CsA) in preventing graft failure after high-risk penetrating keratoplasty (PKP).
Methods
In this retrospective study, 36 eyes of 25 patients who underwent PKP were evaluated. At 24 months post-operatively, the failure rate in the CsA group (n = 19) was compared to the control group (n = 17). For subgroup analysis, the failure rate in the CsA group (n = 9) and control group (n = 9) was compared in patients who underwent a repeat PKP. The patients’ side-effect profile was also collected.
Results
The median follow-up in the CsA group was 32.8 months and 28.9 months in the control group. Graft failure occurred in 31.6% CsA patients and in 68.4% control patients (p = 0.311). In patients with repeat PKP, the failure rate in the CsA group was significantly lower than the control group (22.2% vs. 77.8%, p = 0.018). In one case (5.26%), CsA was discontinued due to gastroinstestinal discomfort.
Conclusions
Low-dose CsA was not beneficial compared to conventional therapy in high-risk PKP patients. However, in the repeat PKP subgroup, the incidence of graft failure was lower with low-dose CsA than with conventional therapy. Although further study is necessary, adding low-dose CsA might be beneficial for repeat PKP patients.
Go to : 
References
1. Luengo-Gimeno F, Tan DT, Mehta JS.Evolution of deep anterior lamellar keratoplasty (DALK). Ocul Surf. 2011; 9:98–110.

2. Lee WB, Jacobs DS, Musch DC. . Descemet's stripping endo-thelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009; 116:1818–30.
3. Keenan TD, Carley F, Yeates D. . Trends in corneal graft sur-gery in the UK. Br J Ophthalmol. 2011; 95:468–72.

4. Williams KA, Lowe M, Bartlett C. . Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008; 86:1720–4.

5. Williams KA, Roder D, Esterman A. . Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology. 1992; 99:403–14.
6. Tan DT, Janardhanan P, Zhou H. . Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008; 115:975–82.e1.
7. Thompson RW Jr, Price MO, Bowers PJ, Price FW Jr.Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003; 110:1396–402.

8. Wagoner MD, Ba-Abbad R, Sutphin JE, Zimmerman MB.Corneal transplant survival after onset of severe endothelial rejection. Ophthalmology. 2007; 114:1630–6.

9. Joseph A, Raj D, Shanmuganathan V. . Tacrolimus im-munosuppression in high-risk corneal grafts. Br J Ophthalmol. 2007; 91:51–5.

10. Chatel MA, Larkin DF.Sirolimus and mycophenolate as combina-tion prophylaxis in corneal transplant recipients at high rejection risk. Am J Ophthalmol. 2010; 150:179–84.

11. Birnbaum F, Mayweg S, Reis A. . Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: long-term re-sults of a prospective, randomised, multicentre study. Eye (Lond). 2009; 23:2063–70.

12. Calne RY, White DJ, Thiru S. . Cyclosporin A in patients re-ceiving renal allografts from cadaver donors. Lancet. 1978; 2:1323–7.

13. Starzl TE, Klintmalm GB, Porter KA. . Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981; 305:266–9.

14. Borghi A, Corazza M, Mantovani L. . Prolonged cyclosporine treatment of severe or recalcitrant psoriasis: descriptive study in a series of 20 patients. Int J Dermatol. 2012; 51:1512–6.

15. Lichtiger S, Present DH, Kornbluth A. . Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994; 330:1841–5.

16. Poon AC, Forbes JE, Dart JK. . Systemic cyclosporin A in high risk penetrating keratoplasties: a case-control study. Br J Ophthalmol. 2001; 85:1464–9.

17. Reinhard T, Sundmacher R, Heering P.Systemic ciclosporin A in high-risk keratoplasties. Graefes Arch Clin Exp Ophthalmol. 1996; 234(Suppl 1):S115–21.

18. Hill JC.The use of systemic cyclosporin a in human corneal trans-plantation: a preliminary report. Doc Ophthalmol. 1986; 62:337–44.

19. Hill JC.Systemic cyclosporine in high-risk keratoplasty. Short-versus long-term therapy. Ophthalmology. 1994; 101:128–33.
20. Reinhard T, Sundmacher R, Godehardt E, Heering P.Preventive systemic cyclosporin A after keratoplasty at increased risk for immune reactions as the only elevated risk factor. Ophthalmologe. 1997; 94:496–500.
21. Shimazaki J, Den S, Omoto M. . Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol. 2011; 152:33–9.e1.

22. Robert PY, Delbosc B, Preux PM. . [Treatment with cyclo-sporin A, with low doses, in high-risk penetrating keratoplasties. A bi-centric study of 90 cases]. J Fr Ophtalmol. 1997; 20:507–14.
23. Boisjoly HM, Tourigny R, Bazin R. . Risk factors of corneal graft failure. Ophthalmology. 1993; 100:1728–35.

24. Kim EC, Kim MS.Graft Rejection in Penetrating Keratoplasty. J Korean Ophthalmol Soc. 2003; 44:289–95.
25. Weisbrod DJ, Sit M, Naor J, Slomovic AR.Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea. 2003; 22:429–34.

26. Woods JE, Anderson CF, DeWeerd JH. . High-dosage intra-venously administered methylprednisolone in renal transplantation. A preliminary report. JAMA. 1973; 223:896–9.

27. Graham RM.Cyclosporine: mechanisms of action and toxicity. Cleve Clin J Med. 1994; 61:308–13.
28. Algros MP, Angonin R, Delbosc B. . Danger of systemic cyclo-sporine for corneal graft. Cornea. 2002; 21:613–4.

29. Patel J, Kobashigawa JA.Minimization of immunosuppression: transplant immunology. Transpl Immunol. 2008; 20(1-2):48–54.
30. Reinhard T, Reis A, Böhringer D. . Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years' results of a randomized prospective clinical trial. Graefes Arch Clin Exp Ophthalmol. 2001; 239:367–72.

31. Rumelt S, Bersudsky V, Blum-Hareuveni T, Rehany U.Systemic cyclosporin A in high failure risk, repeated corneal transplantation. Br J Ophthalmol. 2002; 86:988–92.
32. Völker-Dieben HJ, D'Amaro J, Kok-van Alphen CC. Hierarchy of prognostic factors for corneal allograft survival. Aust N Z J Ophthalmol. 1987; 15:11–8.
33. Fasolo A, Capuzzo C, Fornea M. . Risk factors for graft failure after penetrating keratoplasty: 5-year follow-up from the corneal transplant epidemiological study. Cornea. 2011; 30:1328–35.

34. Anshu A, Lim LS, Htoon HM, Tan DT.Postoperative risk factors influencing corneal graft survival in the Singapore Corneal Transplant Study. Am J Ophthalmol. 2011; 151:442–8.e1.

35. Keown P, Kahan BD, Johnston A. . Optimization of cyclo-sporine therapy with new therapeutic drug monitoring strategies: report from the International Neoral TDM Advisory Consensus Meeting (Vancouver, November 1997). Transplant Proc. 1998; 30:1645–9.

36. Pescovitz MD, Barbeito R. Simulect US01 Study Group. Two-hour post-dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clin Transplant. 2002; 16:378–82.
Go to : 
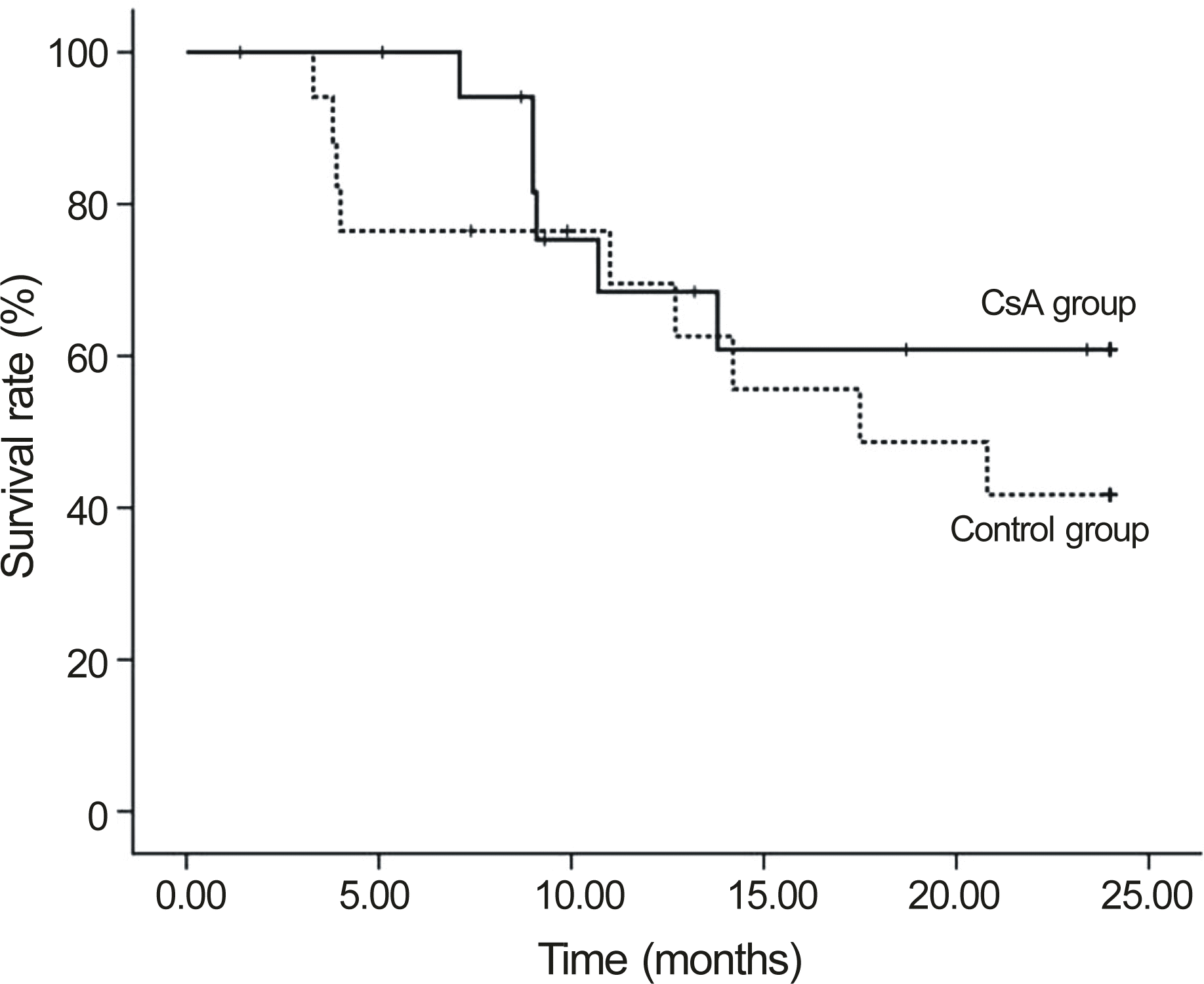 | Figure 1.Comparison of corneal allograft survival between the cyclosporine A and control groups (Kaplan-Meier analysis, Logrank test p = 0.351). |
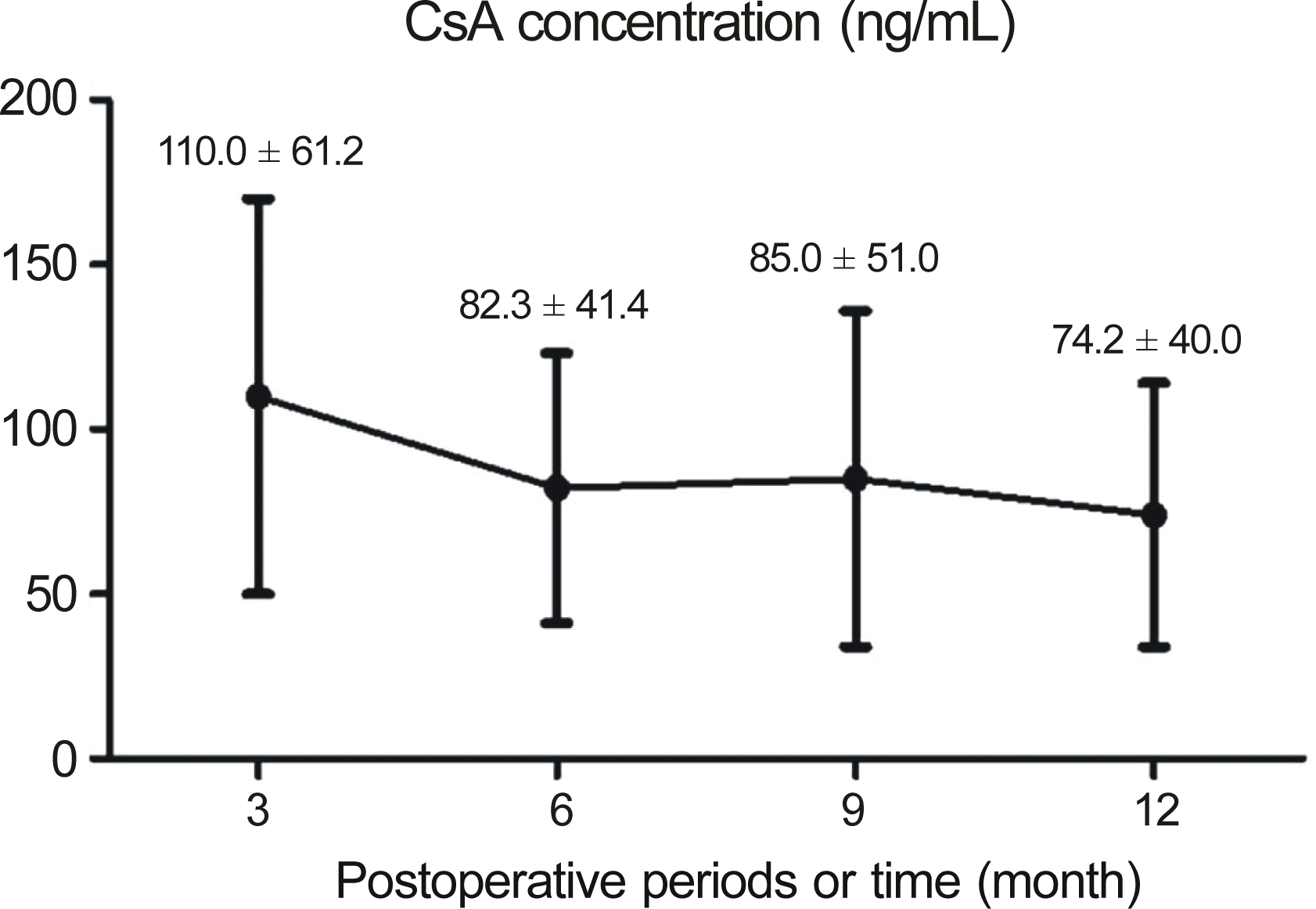 | Figure 2.The graph showing mean blood cyclosporine A con-centration in the low dose systemic cyclosporine A group. |
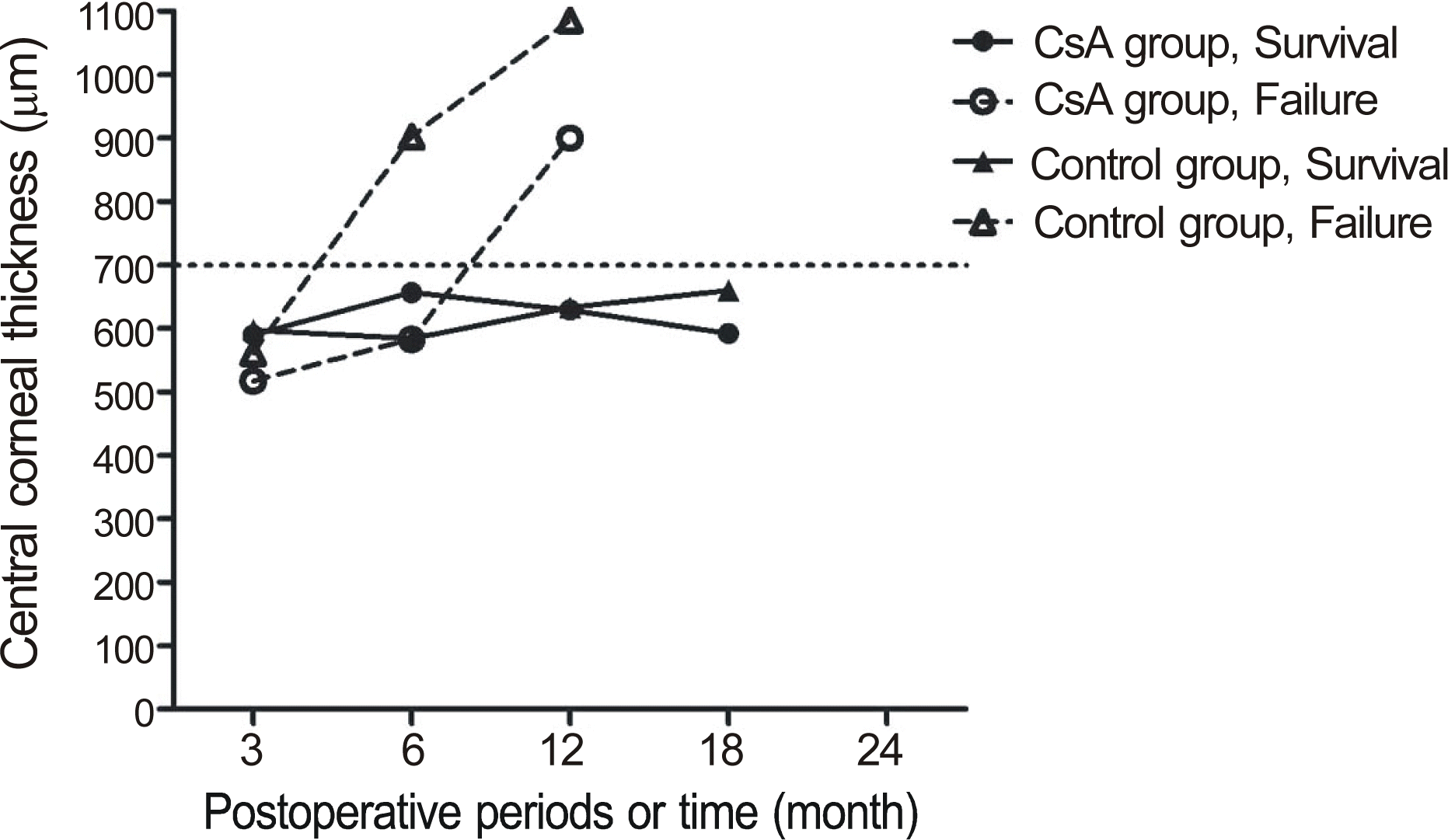 | Figure 3.Change of mean central corneal thickness after penetrating keratoplasty in the cyclosporin A and control groups according to graft survival and failure. |
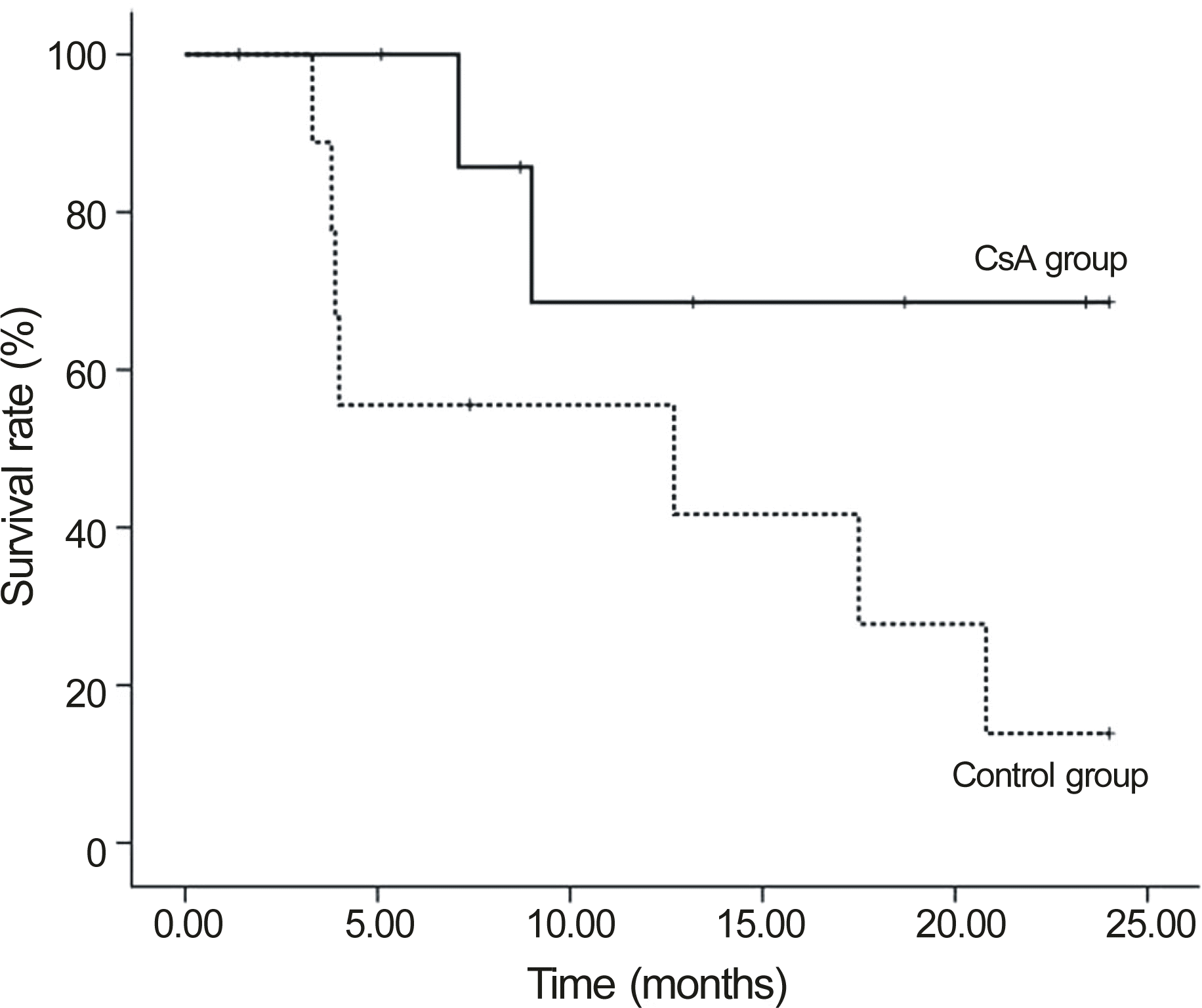 | Figure 4.Comparison of corneal allograft survival between the cyclosporine A and control groups in re-graft cases (Kaplan-Meier analysis, Logrank test p = 0.069). |
Table 1.
Baseline patients characteristics and risk factors for graft failure
| Characteristic | CsA group (n = 19) | Control group (n = 17) | p-value |
|---|---|---|---|
| Age (years) | 62.68 ± 11.40 (31-82) | 60.12 ± 16.38 (26-73) | 0.981* |
| Sex (Male to female ratio) | 18:1 (94.7%) | 12:5 (70.6%) | 0.081† |
| Mean follow up period (months) | 32.14 ± 5.16 | 34.55 ± 13.78 | 0.490* |
| Number of previous grafts | 1.22 ± 0.67 | 1.11 ± 0.33 | 1.000* |
| Primary cause | 0.582† | ||
| Bullous keratopathy | 14 | 9 | |
| Herpes | 2 | 4 | |
| Fungal | 2 | 2 | |
| Bacterial | 1 | 2 |
Table 2.
Baseline patients characteristics and risk factors for graft failure in two or more penetrating keratoplasties
| Characteristic | CsA group (n = 9) | Control group (n = 9) | p-value |
|---|---|---|---|
| Age (years) | 60.44 ± 15.44 (31-82) | 58.67 ± 19.29 (26-73) | 0.965* |
| Sex (Male to female ratio) | 8:1 (88.9%) | 8:1 (88.9%) | 1.000† |
| Mean follow up period (months) | 32.36 ± 5.73 | 35.09 ± 15.66 | 0.605* |
| Number of previous grafts | 1.25 (1-3) | 1.11 (1-2) | 1.000* |
| Primary cause | 0.793† | ||
| Bullous keratopathy | 6 | 4 | |
| Herpes | 2 | 4 | |
| Bacterial | 1 | 1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download