Abstract
Purpose
The cytotoxicities and anti-fibrotic effects of mitomycin C and pirfenidone on human dermal fibroblast were evaluated.
Methods
Initially, 24-hour cell cultures were exposed to transforming growth factor (TGF)-β1, different concentrations of mitomycin C, and pirfenidone solutions in order to evaluate cytotoxicity. Expressions of fibronectin, collagen type 1, α smooth muscle, and β-actin were evaluated by real-time reverse transcription-polymerase chain reaction (RT-PCR) and western blot in mitomycin C solutions at concentrations of 4 μg/mL and 20 μg/mL, and in pirfenidone solutions at 250 μg/mL and 500 μg/mL.
Results
In comparison to cell cultures exposed to TGF-β1 solutions, cytotoxicities were increased in solutions of mitomycin C at 4 μg/mL, 20 μg/mL, 40 μg/mL and pirfenidone at 500 μg/mL, 750 μg/mL, 1,000 μg/mL (p < 0.05, Mann Whitney U-test). The results of real-time RT-PCR show that expressions of fibronectin, collagen type 1, and α smooth muscle were significantly more decreased in all concentrations of mitomycin C and pirfenidone compared to those in TGF-β1 solution. In western blot analysis, expressions of fibronectin and α smooth muscle were decreased in all concentrations of mitomycin C and pirfenidone compared to TGF-β1 solution.
Go to : 
References
1. Carter NJ. Pirfenidone: in idiopathic pulmonary fibrosis. Drugs. 2011; 71:1721–32.
3. Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999; 291:367–73.
4. Hewitson TD, Kelynack KJ, Tait MG, et al. Pirfenidone reduces in vitro rat renal fibroblast activation and mitogenesis. J Nephrol. 2001; 14:453–60.
5. Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol. 1999; 276:L311–8.
6. Oku H, Nakazato H, Horikawa T, et al. Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol. 2002; 446:167–76.
7. Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008; 590:400–8.

8. Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999; 289:211–8.
9. Mitani Y, Sato K, Muramoto Y, et al. Superoxide scavenging activity of pirfenidone-iron complex. Biochem Biophys Res Commun. 2008; 372:19–23.

10. Di Sario A, Bendia E, Svegliati Baroni G, et al. Effect of pirfenidone on rat hepatic stellate cell proliferation and collagen production. J Hepatol. 2002; 37:584–91.

11. Yamazaki T, Yamashita N, Izumi Y, et al. The antifibrotic agent pirfenidone inhibits angiotensin II-induced cardiac hypertrophy in mice. Hypertens Res. 2012; 35:34–40.

12. Zhang S, Shiels IA, Ambler JS, Taylor SM. Pirfenidone reduces fibronectin synthesis by cultured human retinal pigment epithelial cells. Aust NZ J Ophthalmol. 1998; 26(Suppl 1):S74–6.

13. Lin X, Yu M, Wu K, et al. Effects of pirfenidone on proliferation, migration, and collagen contraction of human Tenon's fibroblasts in vitro. Invest Ophthalmol Vis Sci. 2009; 50:3763–70.

14. Kim H, Choi YH, Park SJ, et al. Antifibrotic effect of Pirfenidone on orbital fibroblasts of patients with thyroid-associated ophthalmopathy by decreasing TIMP-1 and collagen levels. Invest Ophthalmol Vis Sci. 2010; 51:3061–6.

15. Zhong H, Sun G, Lin X, et al. Evaluation of pirfenidone as a new postoperative antiscarring agent in experimental glaucoma surgery. Invest Ophthalmol Vis Sci. 2011; 52:3136–42.

16. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003; 48:314–46.

17. Palanca-Capistrano AM, Hall J, Cantor LB, et al. Long-term out- comes of intraoperative 5-fluoro uracil versus intraoperative mito- mycin C in primary trabeculectomy surgery. Ophthalmology. 2009; 116:185–90.
18. Franks WA, Hitchings RA. Complications of 5–fluorouracil after trabeculectomy. Eye (Lond). 1991; 5:385–9.

19. Seong GJ, Park C, Kim CY, et al. Mitomycin-C induces the apoptosis of human Tenon's capsule fibroblast by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest Ophthalmol Vis Sci. 2005; 46:3545–52.
20. Chen T, Kunnavatana SS, Koch RJ. Effects of mitomycin-C on normal dermal fibroblasts. Laryngoscope. 2006; 116:514–7.

21. Anderson MT, Staal FJ, Gitler C, et al. Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc Natl Acad Sci U S A. 1994; 91:11527–31.

22. Yao KS, O'Dwyer PJ. Involvement of NF-kappa B in the induction of NAD(P)H:quinone oxidoreductase (DT-diaphorase) by hypo-xia, oltipraz and mitomycin C. Biochem Pharmacol. 1995; 49:275–82.
23. Baldassarre F, Mallardo M, Mezza E, et al. Regulation of NF-kappa B through the nuclear processing of p105 (NF-kappa B1) in Epstein-Barr virus-immortalized B cell lines. J Biol Chem. 1995; 270:31244–8.
Go to : 
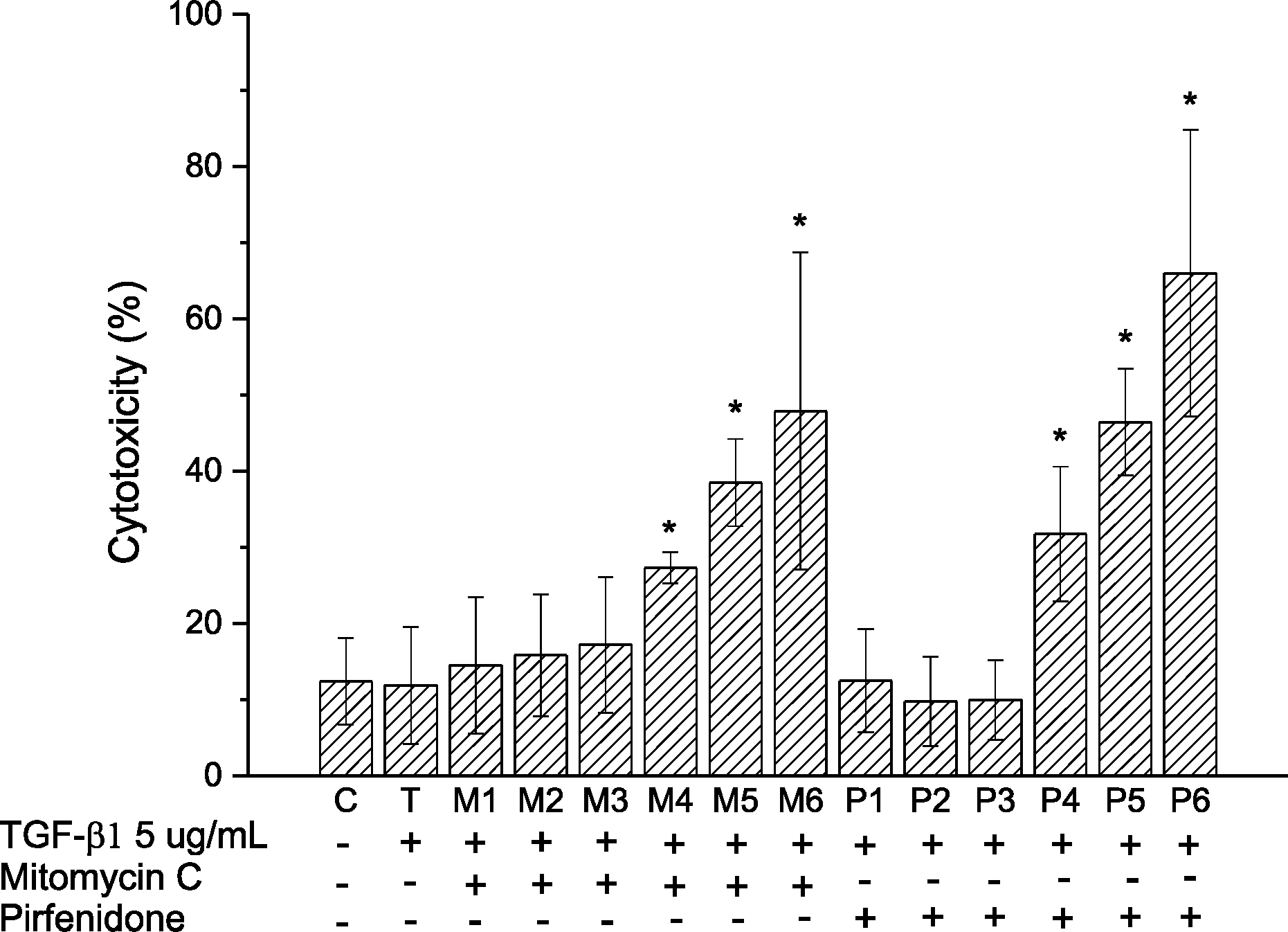 | Figure 1.Result of lactate dehydrogenase (LDH) assay. M4, M5, M6, P4, P5, P6 solutions increased cytotoxicity significantly (*p < 0.05, Mann Whitney U-test). Sample C: only Dulbecco's modified eagle's medium (DMEM); T-P6: add transforming growth factor (TGF)-βl 5 ng/mL; M1-M6: add Mitomycin C, 0.00004 (Ml), 0.004 (M2), 0.4 (M3), 4 (M4), 20 (M5), 40 μg/mL (M6; P1-P6: add Pirfenidone, 10 (Pl), 100 (P2), 250 (P3), 500 (P4), 750 (P5), 1,000 μg/mL (P6). TGF = transforming growth factor; C = control; T = TGF-β 1; M = mitomycin C; P = pirfenidone. |
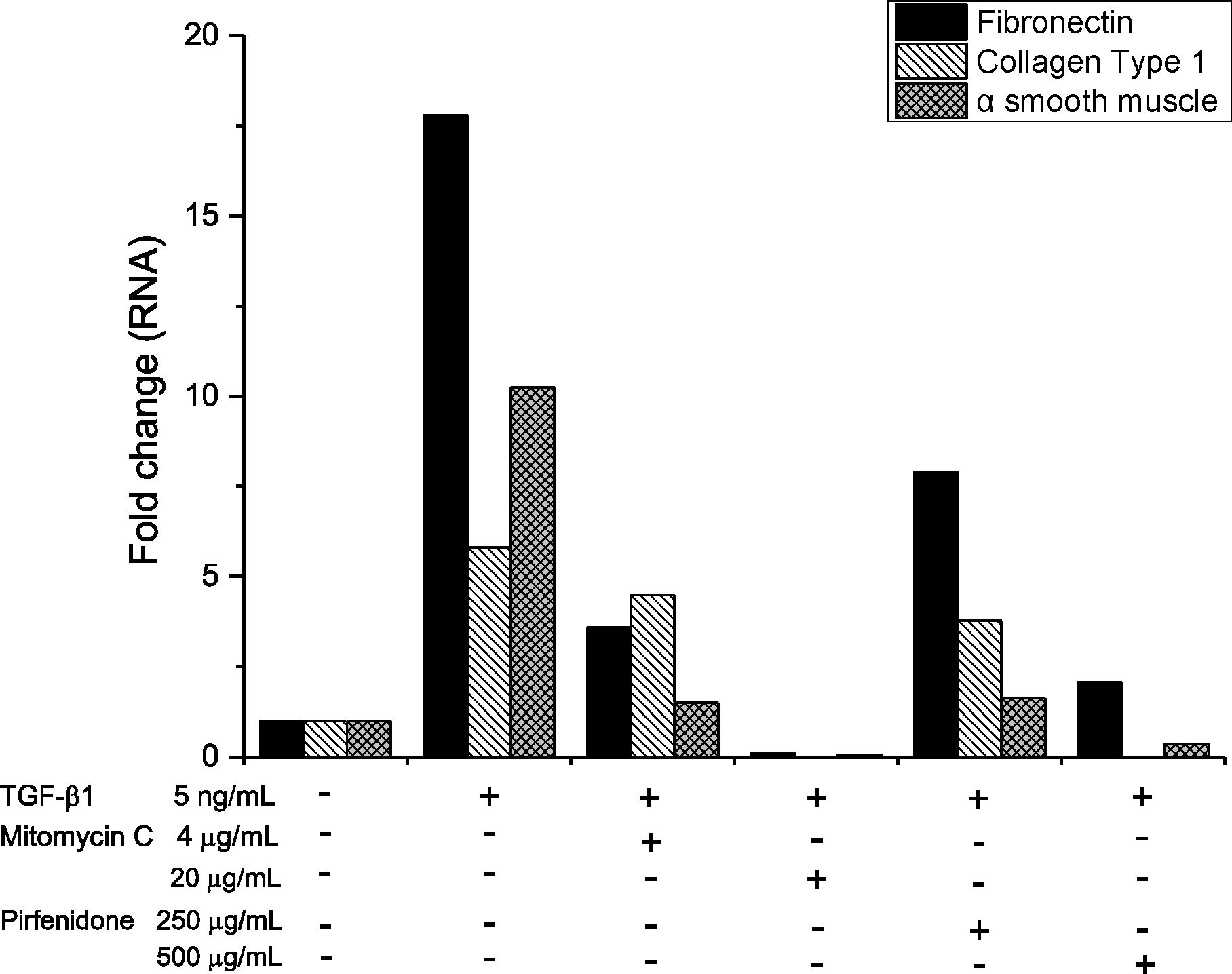 | Figure 2.Result of real time reverse transcription polymerase chain reaction (RT-PCR). Mitomycin C and pirfenidone solution decreased ribonucleic acid (RNA) expression of fibronectin, collagen I, α smooth muscle, which compared to the only transforming growth factor (TGF)-β1 solution. |
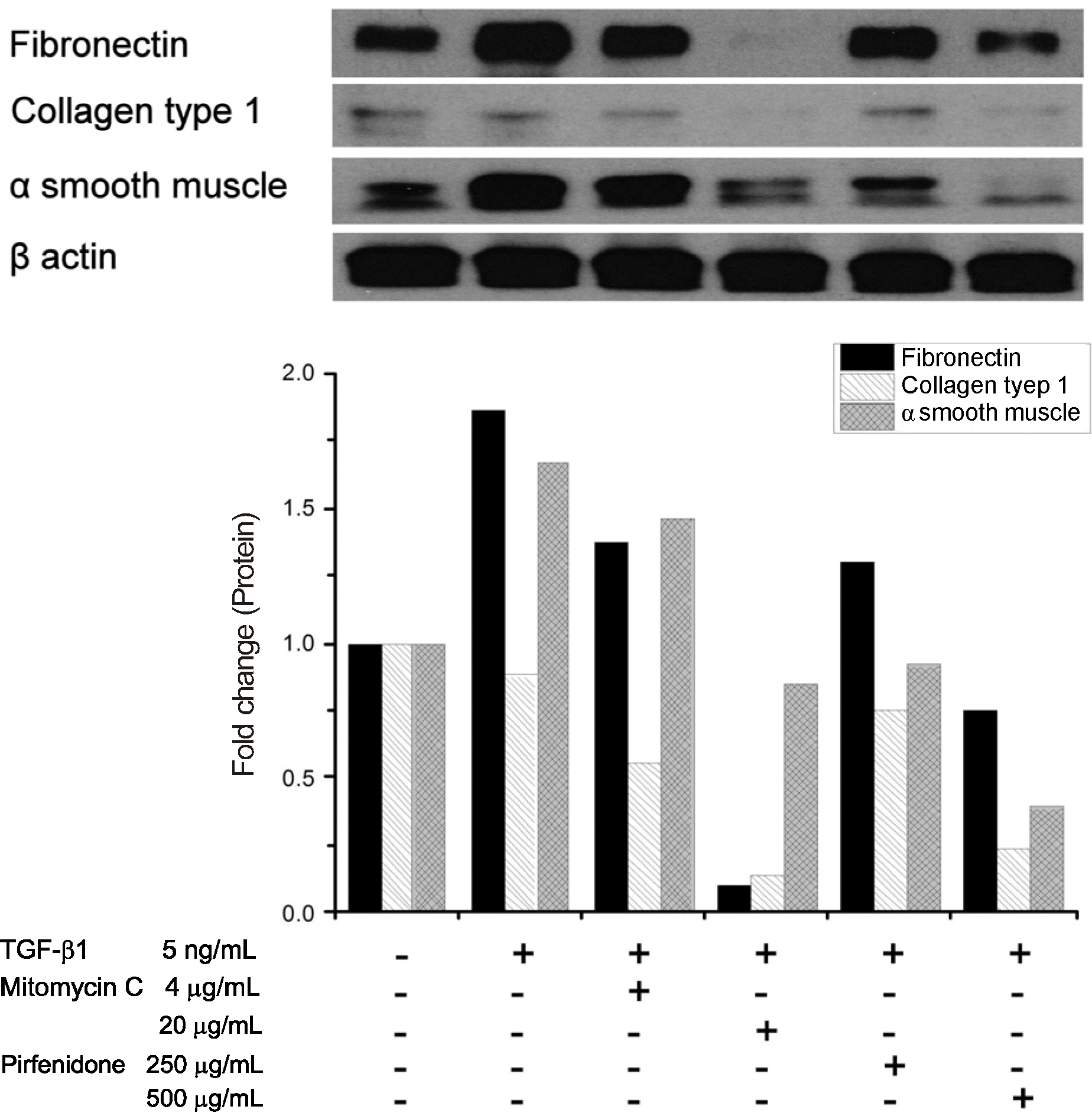 | Figure 3.Result of western blot. Mitomycin C and pirfenidone solution decreased protein expression of fibronectin, α smooth muscle, which compared to the only transforming growth factor (TGF)-β1 solution. Protein expression of collagen type I was too weak. |
Table 1.
Primer sequences for real time reverse transcription polymerase chain reaction (RT-PCR)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download