Abstract
Purpose
To compare the biomechanical properties of keratoconus, keratoconus suspect, and normal subjects measured by ocular response analyzer.
Methods
A total of 72 patients were divided into 3 groups: keratoconus, suspected keratoconus, and normal control subjects. The 3 age-matched groups were evaluated according to age, sex, and visual acuity. Slit lamp examinations, Pentacam, and ocular response analyzer (ORA) examinations were performed. Mean corneal refractive power, central corneal thickness, corneal hysteresis (CH), and corneal resistance factor (CRF) were evaluated and analyzed.
Results
Twenty-four eyes were included in each group. The mean age was 23.8 years in keratoconus, 26.0 years in sus-pected keratoconus and 26.1 years in normal subject groups. Mean corneal refractive power was significantly higher in keratoconus (p < 0.001) and suspected keratoconus (p = 0.001) groups than in the normal subject group. Mean central corneal thickness showed significant differences among the 3 groups (p < 0.05). CH was significantly lower in keratoconus than suspected keratoconus (p = 0.025) and normal subject groups (p = 0.005), but showed no significant difference be-tween suspected keratoconus and normal subject groups. CRF showed significant differences among all 3 groups (p < 0.05). CH and CRF had negative correlations with mean corneal refractive power and positive correlations with central cor-neal thickness.
Conclusions
CH and CRF measured by ORA were significantly different between keratoconus and normal subject groups and had significant correlations with mean corneal refractive power and central corneal thickness. CRF may be a useful method to differentiate between suspected keratoconus and normal cornea patients.
Go to : 
References
2. Lapid-Gortzak R, Rosen S, Weitzman S, Lifshitz T. Videokeratography findings in children with vernal keratoconjunctivitis versus those of healthy children. Ophthalmology. 2002; 109:2018–23.

3. Maeda N, Klyce SD, Smolek MK, Thompson HW. Automated ker-atoconus screening with corneal topography analysis. Invest Ophthalmol Vis Sci. 1994; 35:2749–57.
4. Dastjerdi MH, Hashemi H. A quantitative corneal topography in-dex for detection of keratoconus. J Refract Surg. 1998; 14:427–36.

5. Rabinowitz YS, Rasheed K. KISA% index: a quantitative video-keratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999; 25:1327–35.

6. Dupps WJ Jr.Hysteresis: new mechanospeak for the ophthalmologist. J Cataract Refract Surg. 2007; 33:1499–501.

7. Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005; 31:156–62.

8. Galletti JG, Pförtner T, Bonthoux FF. Improved keratoconus de-tection by ocular response analyzer testing after consideration of corneal thickness as a confounding factor. J Refract Surg. 2012; 28:202–8.

9. Kim SW, Seo SG, Her J, Park SJ. Factors affecting the ocular re-sponse analyzer parameters in normal Korean. J Korean Ophthalmol Soc. 2009; 50:1605–10.

10. Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye. 2007; 30:223–32.

11. Piñero DP, Alió JL, Alesón A. . Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in sub-clinical and different stages of clinical keratoconus. J Cataract Refract Surg. 2010; 36:814–25.

12. Schlegel Z, Hoang-Xuan T, Gatinel D. Comparison of and correlation between anterior and posterior corneal elevation maps in normal eyes and keratoconus eyes. J Cataract Refract Surg. 2008; 34:789–95.
13. Krumeich JH, Daniel J, Knülle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998; 24:456–63.

14. Krachmer JH, Feder RS, Belin MW. Keratoconus and related non-inflammatory corneal thinning disorders. Surv Ophthalmol. 1984; 28:293–322.

15. Edrington TB, Barr JT, Zadnik K. . Standardized rigid contact lens fitting protocol for keratoconus. Optom Vis Sci. 1996; 73:369–75.

16. Mandell RB, Polse KA. Keratoconus: spatial variation of corneal thickenss as a diagnostic test. Arch Ophthalmol. 1969; 82:182–8.
17. Rabinowitz YS, Rasheed K, Yang H, Elashoff J. Accuracy of ultra-sonic pachymetry and videokeratography in detecting keratoconus. J Cataract Refract Surg. 1998; 24:196–201.

18. Ambekar R, Toussaint KC Jr, Wagoner , Johnson A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J Mech Behav Biomed Mater. 2011; 4:223–36.

19. Edmund C. Corneal elasticity and ocular rigidity in normal and keratoconic eyes. Acta Ophthalmol (Copenh). 1988; 66:134–40.

20. Andreassen TT, Simonsen AH, Oxlund H. Biomechanical proper-ties of keratoconus and normal corneas. Exp Eye Res. 1980; 31:435–41.

21. Akhtar S, Bron AJ, Salvi SM. . Ultrastructural analysis of col-lagen fibrils and proteoglycans in keratoconus. Acta Ophthalmol. 2008; 86:764–72.

Go to : 
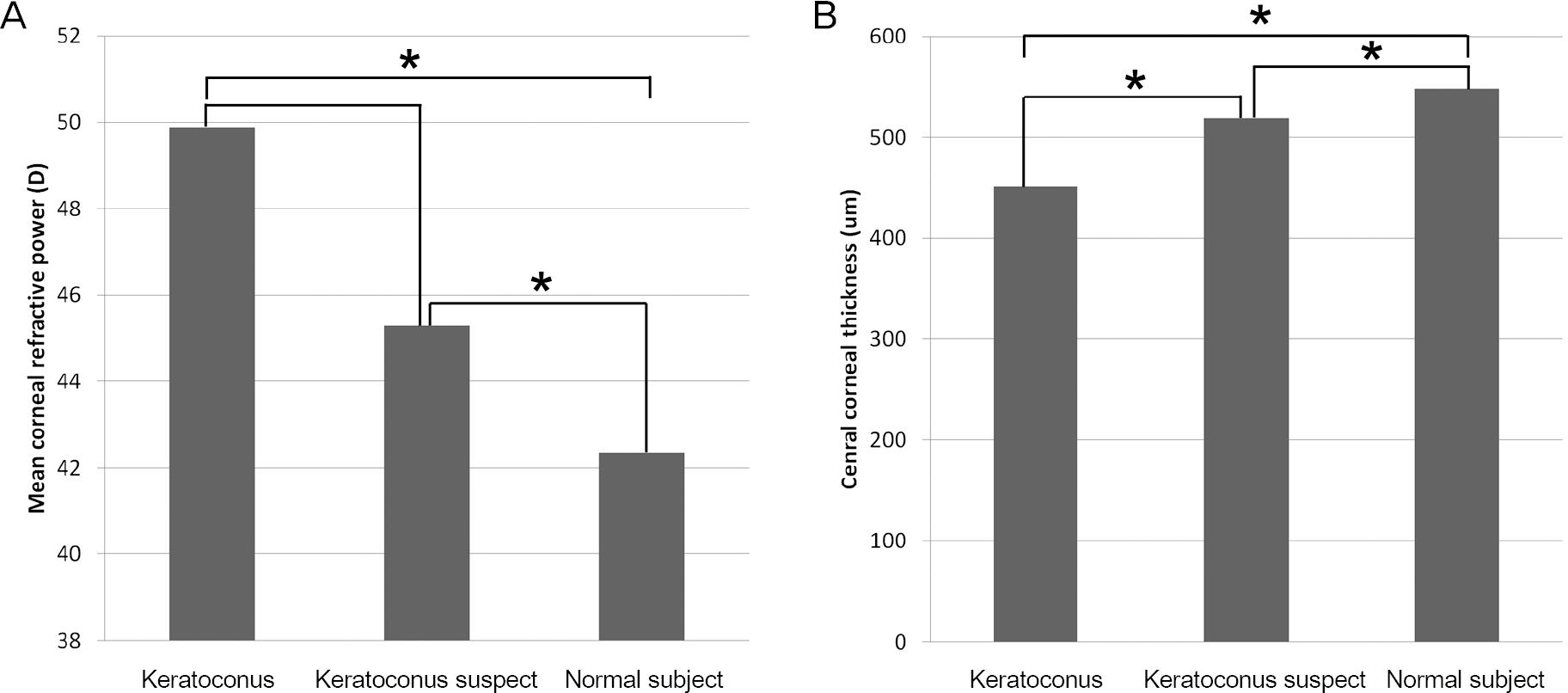 | Figure 1.Comparison of mean corneal refractive power (A) and central corneal thickness (B) among keratoconus, keratoco-nus suspect, and normal control groups. * Indicates a pair of groups that has a significant difference between each other with p < 0.05. |
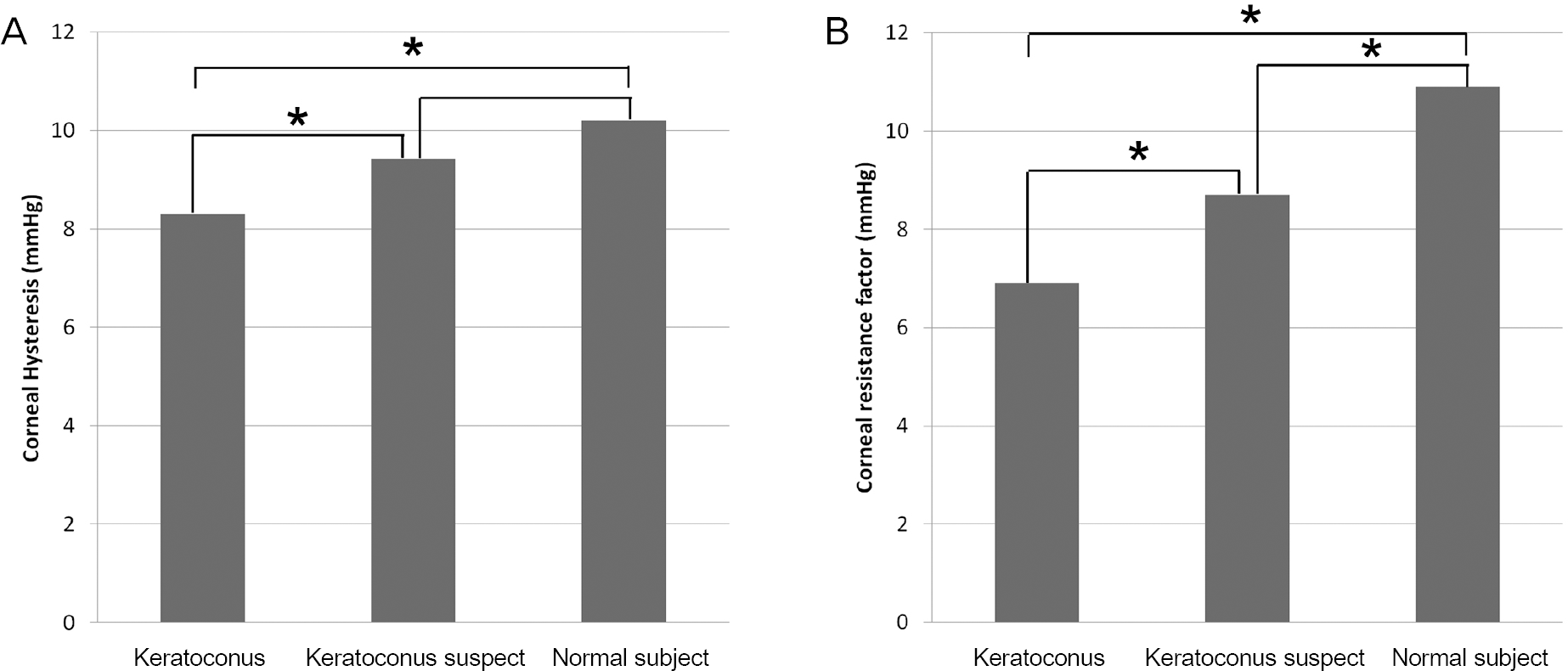 | Figure 2.Comparison of corneal hysteresis (A) and corneal resistance factor (B) among keratoconus, keratoconus suspect, and normal control groups. * Indicates a pair of groups that has a significant difference between each other with p < 0.05. |
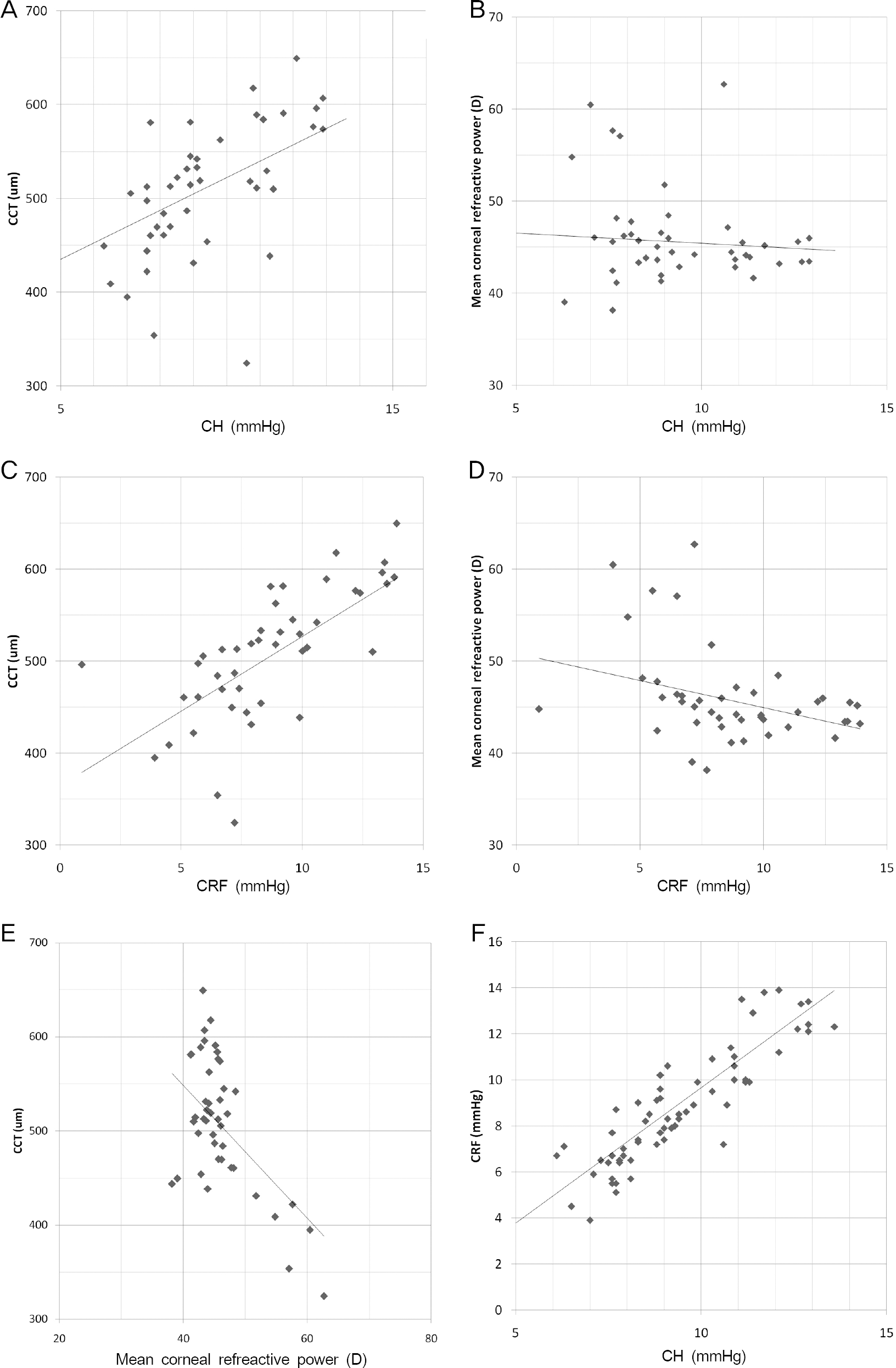 | Figure 3.Parameters significantly correlated with each other. (A: Corneal hysteresis (CH) to central corneal thickness (CCT), B: CH to mean corneal refractive power, C: Corneal resistance factor (CRF) to CCT, D: CRF to mean corneal refractive power, E: mean corneal refractive power to CCT, F: CH to CRF). A, C, F showed positive correlations and B, D, E showed negative correlations. |
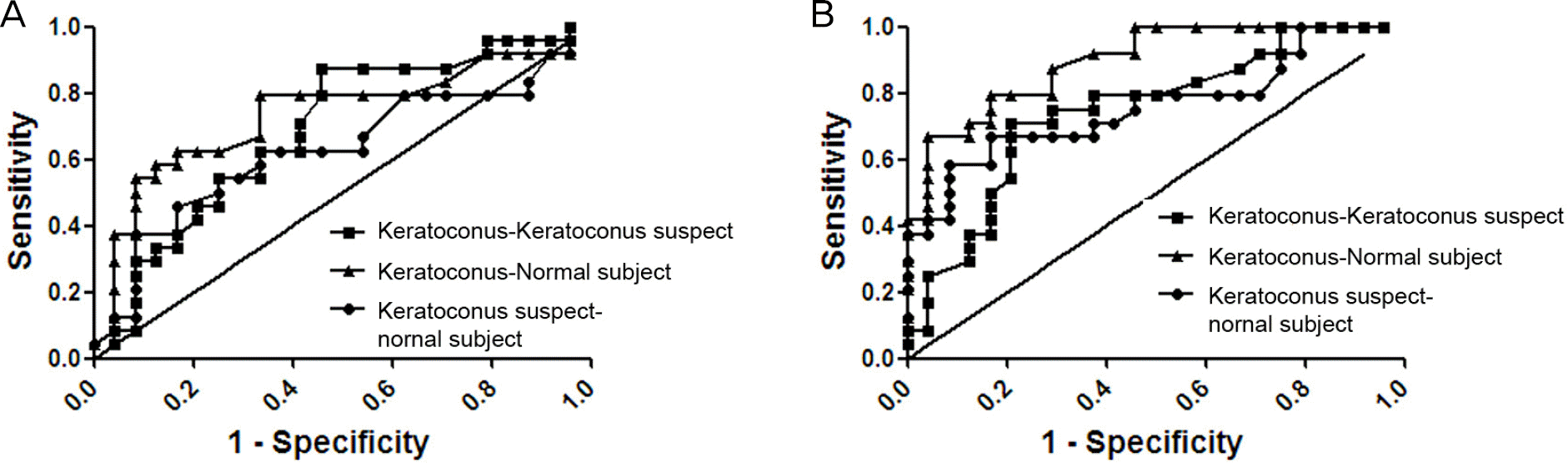 | Figure 4.The receiver operating characteristic (ROC) graph showing the sensitivity and specificity of the corneal hysteresis (A) and corneal resistance factor (B) for each pair of groups. |
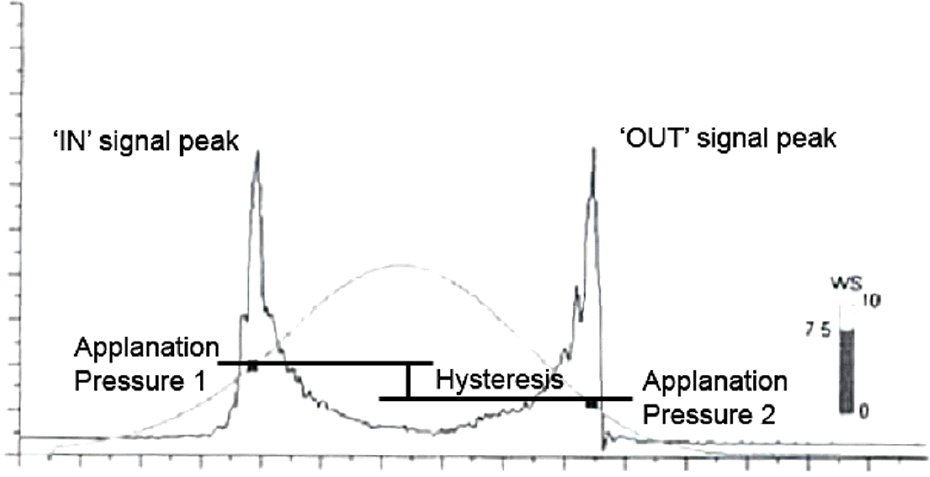 | Figure 5.Measurement of corneal hysteresis using Ocular Response Analyzer (Reichert Inc., Depew, NY). It is derived from the difference between the inward applanation and out-ward applanation. |
Table 1.
Baseline characteristics of patients in keratoconus, keratoconus suspect, and control subjects




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download