Abstract
Purpose
To investigate the efficacy of combined treatment with 0.05% cyclosporine A and 20% umbilical cord serum eye drops for severe dry eye associated with graft-versus-host disease (GVHD).
Methods
Eighteen patients with severe dry eye associated with GVHD were treated with 0.05% cyclosporine A and 20% umbilical cord serum eye drops (group 1, n = 16), 0.05% cyclosporine A eye drops (group 2, n = 10) or artificial tears only (group 3, n = 10). Tear film break up time (BUT), Schirmer test, tear clearance rate (TCR), and keratoepithelio pathy score were measured before and 1, 3 and 6 months after treatment.
Results
In group 1, significant improvement was observed in tear film BUT (from 3.25 ± 1.18 s to 6.63 ± 0.96 s, p < 0.01), TCR (from 2.38 ± 0.72 to 3.13 ± 0.72, p < 0.01) and keratoepithelio pathy score (from 6.31 ± 2.15 to 0.88 ± 0.89, p < 0.01) 6 months after treatment. Compared with group 2 and group 3, group 1 showed significant improvement in BUT (3 and 6 months after treatment) and keratoepitheliopathy score (1, 3 and 6 months after treatment).
References
1. Horowitz MM. Uses and growth of hematopoietic cell transplantation.In : Blume KG, Forman SJ, Applebaum FR, editors. Thomas' Hematopoietic Cell Transplantation. 3rd ed.Blackwell: Malden, MA;2004. v. 1. chap. 2.
2. Martin PJ. Overview of hematopoietic cell transplantation immunology. In : Blume KG, Forman SJ, Applebaum FR, editors. Thomas' Hematopoietic Cell Transplantation. 3rd ed.Blackwell;Malden, MA: 2004. v. 1. chap. 3.
3. Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003; 22:S19–27.

4. Franklin RM, Kenyon KR, Tutschka PJ, et al. Ocular manifestations of graft-vs-host disease. Ophthalmology. 1983; 90:4–13.

5. Ogawa Y, Okamoto S, Wakui M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999; 83:1125–30.

6. Hirst LW, Jabs DA, Tutschka PJ, Green WR, Santos GW. The eye in bone marrow transplantation. I. Clinical study. Arch Ophthalmol. 1983; 101:580–4.

7. Anderson NG, Regillo C. Ocular manifestations of graft versus host disease. Curr Opin Ophthalmol. 2004; 15:503–7.

8. Johnson DA, Jabs DA. The ocular manifestations of graft-ver-sus-host disease. Int Ophthalmol Clin. 1997; 37:119–33.
9. Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003; 31:579–83.

10. Murphy PT, Sivakumaran M, Fahy G, Hutchinson RM. Successful use of topical retinoic acid in severe dry eye due to chronic graft-versus-host disease. Bone Marrow Transplant. 1996; 18:641–2.
11. Rocha EM, Pelegrino FS, de Paiva CS, et al. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant. 2000; 25:1101–3.

12. Ogawa Y, Okamoto S, Kuwana M, et al. Successful treatment of dry eye in two patients with chronic graft-versus-host disease with systemic administration of FK506 and corticosteroids. Cornea. 2001; 20:430–4.

13. Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000; 19:644–9.
14. Nussenblatt RB, Palestine AG. Cyclosporine: immunology, pharmacology and therapeutic uses. Surv Ophthalmol. 1986; 31:159–69.

15. Hemady R, Tauber J, Foster CS. Immunosuppressive drugs in immune and inflammatory ocular disease. Surv Ophthalmol. 1991; 35:369–85.

16. Jabs DA, Wingard J, Green WR, et al. The eye in bone marrow transplantation. III. Conjunctival graft-vs-host disease. Arch Ophthalmol. 1989; 107:1343–8.

17. Bhan AK, Fujikawa LS, Foster CS. T-cell subsets and Langerhans cells in normal and diseased conjunctiva. Am J Ophthalmol. 1982; 94:205–12.

18. Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol. 2005; 89:1363–7.

19. Lelli GJ Jr, Musch DC, Gupta A, et al. Ophthalmic cyclosporine use in ocular GVHD. Cornea. 2006; 25:635–8.

20. Wang Y, Ogawa Y, Dogru M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2008; 41:293–302.

21. Lee SH, Im SK, Woo JM, Yoon KC. Long-term evaluation after topical cyclosporine treatment in dry eye patients with graft-versus-host disease. J Korean Ophthalmol Soc. 2009; 50:27–33.

22. Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001; 85:1188–97.

23. Yoon KC, Im SK, Park YG, et al. Application of umbilical cord serum eyedrops for the treatment of dry eye syndrome. Cornea. 2006; 25:268–72.

24. Yoon KC, You IC, Im SK, et al. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007; 114:1637–42.

25. Yoon KC, Heo H, Jeong IY, Park YG. Therapeutic effect of umbilical cord serum eyedrops for persistent corneal epithelial defect. Korean J Ophthalmol. 2005; 19:174–8.

26. Yoon KC, Jeong IY, Im SK, et al. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007; 39:231–5.

27. Vajpayee RB, Mukerji N, Tandon R, et al. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. Br J Ophthalmol. 2003; 87:1312–6.

28. Sharma N, Goel M, Velpandian T, et al. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011; 52:1087–92.

29. Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005; 112:1790–4.

30. Djalilian AR, Nussenblatt RB, Holland EJ. Immunosuppressive therapy in ocular surface transplantation. In : Holland EJ, Mannis MJ, editors. Ocular Surface Disease Medical and Surgical Management. 1st ed.New York: Springer;2002. v. 1. chap. 22.
31. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001; 108:568–92.

33. Kaido M, Goto E, Dogru M, Tsubota K. Punctal occlusion in the management of chronic Stevens-Johnson syndrome. Ophthalmology. 2004; 111:895–900.

34. Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980; 69:204–17.

35. Mencucci R, Rossi Ferrini C, Bosi A, et al. Ophthalmological aspects in allogenic bone marrow transplantation: Sjögren-like syndrome in graft-versus-host disease. Eur J Ophthalmol. 1997; 7:13–8.

36. Tichelli A, Duell T, Weiss M, et al. Late-onset keratoconjunctivitis sicca syndrome after bone marrow transplantation: incidence and risk factors. European Group or Blood and Marrow Transplantation (EBMT) Working Party on Late Effects. Bone Marrow Transplant. 1996; 17:1105–11.
37. Ogawa Y, Kuwana M, Yamazaki K, et al. Periductal area as the primary site for T-cell activation in lacrimal gland chronic graft-versus-host disease. Invest Ophthalmol Vis Sci. 2003; 44:1888–96.

38. Ogawa Y, Yamazaki K, Kuwana M, et al. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci. 2001; 42:111–9.
39. del Castillo JM, de la Casa JM, Sardiña RC, et al. Treatment of recurrent corneal erosions using autologous serum. Cornea. 2002; 21:781–3.

40. Tsubota K, Higuchi A. Serum application for the treatment of ocular surface disorders. Int Ophthalmol Clin. 2000; 40:113–22.

41. Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004; 88:647–52.

42. Tananuvat N, Daniell M, Sullivan LJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001; 20:802–6.

43. Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984; 27:459–61.

44. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren's syndrome. Br J Ophthalmol. 1999; 83:390–5.

45. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. 2007; Ocul Surf. 2007; 5:163–78.
Figure 1.
Comparison of tear film break up time (A), schirmer test (B), tear clearance rate (C), and keratoepitheliopathy score (D) between the groups. *Statistically difference between group 1 and group 2, †Statistically difference between group 1 and group 3, ‡Statistically difference between group 2 and group 3
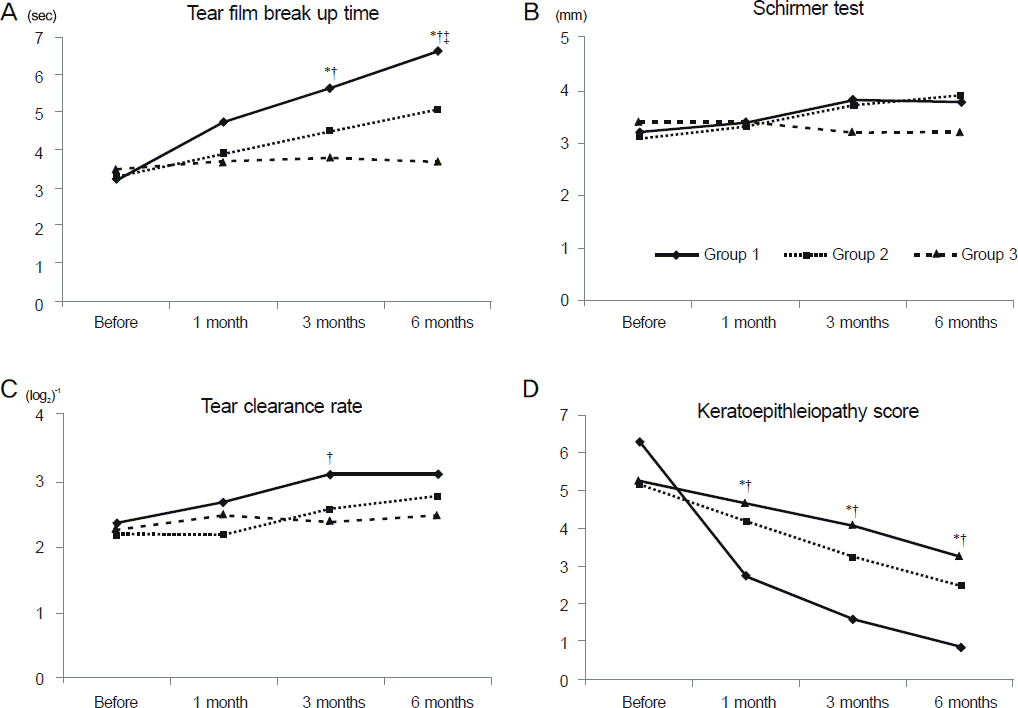
Table 1.
Characteristics of patients with severe dry eye associated with graft-versus-host disease
Table 2.
Tear film and ocular surface change after treatment with cyclosporine A and umbilical cord serum eye drops in severe dry eye associated with graft-versus-host disease
Table 3.
Tear film and ocular surface change after treatment with cyclosporine A eye drops in severe dry eye associated with graft-versus-host disease
Table 4.
Tear film and ocular surface change after treatment with 0.1% sodium hyaluronate eye drops in severe dry eye associated with graft-versus-host disease




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download