Abstract
Purpose
To evaluate the effect and safety of subconjunctival bevacizumab injection immediately after primary pterygium surgery.
Methods
From October 2010 to June 2011, 54 patients (54 eyes) with primary pterygium who had received pterygium excision with the bare sclera technique were evaluated. Twenty-seven patients (27 eyes) in the bevacizumab group received a subconjunctival injection of 5 mg (0.2 ml) bevacizumab and 27 patients (27 eyes) in the control group received a subconjunctival injection of 0.2 ml balanced salt solution immediately after surgery. At the 6-month follow-up, the degree of fibrovascular tissue proliferation, the recurrence rate of pterygium and the effect of wound healing were analyzed prospectively.
Results
One month after the surgery, the degree of fibrovascular tissue proliferation was inhibited in the bevacizumab group compared to the control group (p = 0.028). However, 3 to 6 months after surgery, there was no significant difference between the 2 groups. In addition, there was no significant difference between the 2 groups in the recurrence rate of pterygium and wound healing after surgery.
Go to : 
References
1. Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007; 18:308–13.

2. Todani A, Melki SA. Pterygium: current concepts in pathogenesis and treatment. Int Ophthalmol Clin. 2009; 49:21–30.
3. Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001; 42:1963–8.
4. Lee DH, Cho HJ, Kim JT, et al. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001; 20:738–42.

5. Marcovich AL, Morad Y, Sandbank J, et al. Angiogenesis in abdominal: morphometric and immunohistochemical study. Curr Eye Res. 2002; 25:17–22.
6. Razeghinejad MR, Hosseini H, Ahmadi F, et al. Preliminary results of subconjunctival bevacizumab in primary pterygium excision. Ophthalmic Res. 2010; 43:134–8.

7. Shenasi A, Mousavi F, Shoa-Ahari S, et al. Subconjunctival abdominal immediately after excision of primary pterygium: the first clinical trial. Cornea. 2011; 30:1219–22.
8. Lee JW, Park YJ, Kim IT, Lee KW. Clinical results after application of bevacizumab in recurrent pterygium. J Korean Ophthalmol Soc. 2008; 49:1901–9.

9. Teng CC, Patel NN, Jacobson L. Effect of subconjunctival abdominal on primary pterygium. Cornea. 2009; 28:468–70.
10. Banifatemi M, Razeghinejad MR, Hosseini H, Gholampour A. Bevacizumab and ocular wound healing after primary pterygium excision. J Ocul Pharmacol Ther. 2011; 27:17–21.

11. Frucht-Pery J, Raiskup F, Ilsar M, et al. Conjunctival autografting combined with low-dose mitomycin C for prevention of primary pterygium recurrence. Am J Ophthalmol. 2006; 141:1044–50.

12. Sarnicola V, Vannozzi L, Motolese PA. Recurrence rate using abdominal glue-assisted ipsilateral conjunctival autograft in pterygium surgery: 2-year follow-up. Cornea. 2010; 29:1211–4.
13. Mery G, Maalouf T, George JL, et al. [Limbal-conjunctival autograft in pterygium surgery]. J Fr Ophthalmol. 2010; 33:92–8.
14. Lei G. Surgery for pterygium using a conjunctival pedunculated flap slide. Br J Ophthalmol. 1996; 80:33–4.

15. Dadeya S, Malik KP, Gullian BP. Pteryguim surgery: conjunctival rotation autograft versus conjunctival autograft. Ophthalmic Surg Lasers. 2002; 33:269–74.
16. Dupps WJ Jr, Jeng BH, Meisler DM. Narrow-strip conjunctival abdominal for treatment of pterygium. Ophthalmology. 2007; 114:227–31.
17. Ozer A, Yildirim N, Erol N, et al. abdominal results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft abdominals in primary pterygium excisions. Ophthalmologica. 2009; 223:269–73.
18. Jain AK, Bansal R, Sukhija J. Human amniotic membrane abdominal with fibrin glue in management of primary pterygia: a new tuck-in technique. Cornea. 2008; 27:94–9.
19. Akinci A, Zilelioglu O. Comparison of limbal-conjunctival abdominal and intraoperative 0.02% mitomycin-C for treatment of abdominal pterygium. Int Ophthalmol. 2007; 27:281–5.
20. Young AL, Leung GY, Wong AK, et al. A randomised trial abdominal 0.02% mitomycin C and limbal conjunctival autograft after abdominal of primary pterygium. Br J Ophthalmol. 2004; 88:995–7.
21. Rubinfeld RS, Pfister RR, Stein RM, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992; 99:1647–54.

22. Manning CA, Kloess PM, Diaz MD, Yee RW. Intraoperative abdominal in primary pterygium excision. A prospective, randomized trial. Ophthalmology. 1997; 104:844–8.
23. Oguz H, Basar E, Gurler B. Intraoperative application versus abdominal mitomycin C eye drops in pterygium surgery. Acta Ophthalmol Scand. 1999; 77:147–50.
24. Prabhasawat P, Tesavibul N, Leelapatranura K, et al. Efficacy of subconjunctival 5-fluorouracil and triamcinolone injection in abdominal recurrent pterygium. Ophthalmology. 2006; 113:1102–9.
25. Dadeya S, Kamlesh . Intraoperative daunorubicin to prevent the recurrence of pterygium after excision. Cornea. 2001; 20:172–4.

26. Bekibele CO, Baiyeroju AM, Ajayi BG. 5-fluorouracil vs. beta-irradiation in the prevention of pterygium recurrence. Int J Clin Pract. 2004; 58:920–3.

27. Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008; 88:579–90.

28. Modarres M, Naseripour M, Falavarjani KG, et al. Intravitreal abdominal of 2.5 mg versus 1.25 mg bevacizumab (Avastin) for abdominal of CNV associated with AMD. Retina. 2009; 29:319–24.
29. Jorge R, Costa RA, Calucci D, et al. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina. 2006; 26:1006–13.

30. Iliev ME, Domig D, Wolf-Schnurrbursch U, et al. Intravitreal abdominal (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006; 142:1054–6.
31. Dorta P, Kychenthal A. Treatment of type 1 retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina. 2010; 30:S24–31.

32. Kim SW, Ha BJ, Kim EK, et al. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008; 115:e33–8.

33. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular abdominal of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008; 146:508–12.
34. Saxena S, Vishwkarma K, Khattri M, Kishore P. Multiple subabdominal bevacizumab for advanced primary pterygium. Ann Ophthalmol (Skokie). 2010; 42:28–30.
35. Jeong JH, Chun YS, Kim JC. The effects of a subtenoncapsular abdominal of bevacizumab for ocular surface disease with corneal neovascularization. J Korean Ophthalmol Soc. 2009; 50:1475–82.
Go to : 
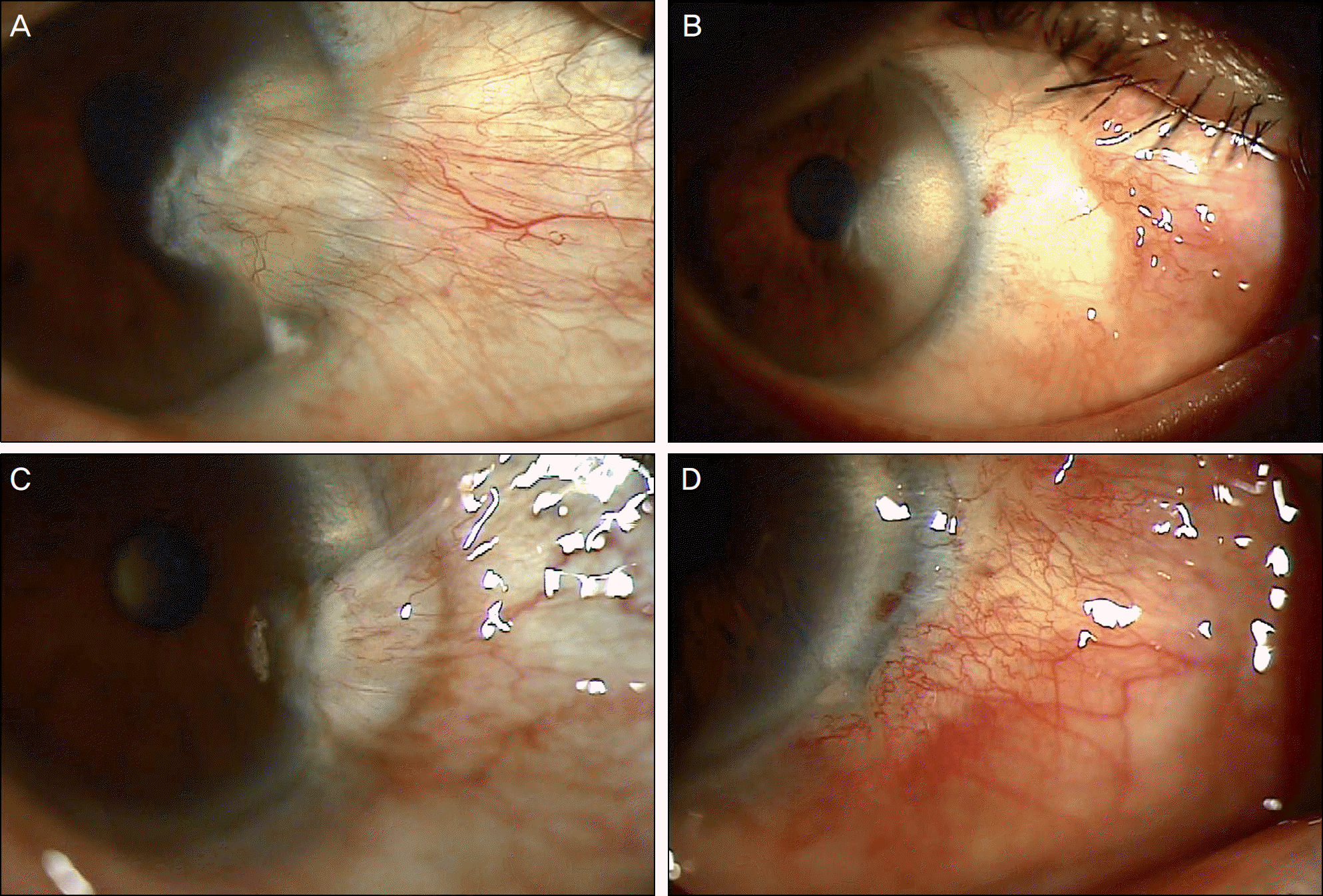 | Figure 1.(A) Bevacizumab group, preoperative appearance. (B) Bevacizumab group, One month after surgery. The proliferation of fibrovascular tissue is inhibited (grade 0). (C) Control group, preoperative appearance. (D) Control group, One month after surgery. The proliferation of fibrovascular tissue is found (grade 1). |
Table 1.
Demographic characteristics of the patients
Table 2.
Postoperative courses in the bevacizumab group and control group
Table 3.
Postoperative fibrovascular proliferation grade
| Bevacizumab group (n) | Control group (n) | p-value | |
|---|---|---|---|
| Postoperative 1 wk | |||
| Grade 0 | 27 | 27 | |
| Grade 1* | 0 | 0 | 1 |
| Grade 2* | 0 | 0 | |
| Postoperative 1 mon | |||
| Grade 0 | 20 | 12 | |
| Grade 1 | 7 | 15 | 0.028 |
| Grade 2* | 0 | 0 | |
| Postoperative 3 mons | |||
| Grade 0 | 10 | 9 | |
| Grade 1 | 15 | 17 | 0.996 |
| Grade 2* | 2 | 1 | |
| Postoperative 6 mons | |||
| Grade 0 | 7 | 5 | |
| Grade 1 | 14 | 14 | 0.437 |
| Grade 2* | 6 | 8 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download