Abstract
Purpose
Pantothenate kinase-associated neurodegeneration (PKAN), also known as neurodegeneration with brain iron accumulation is an extremely rare degenerative disease. The present study reports a case of retinal pigmentary changes in PKAN.
Case summary
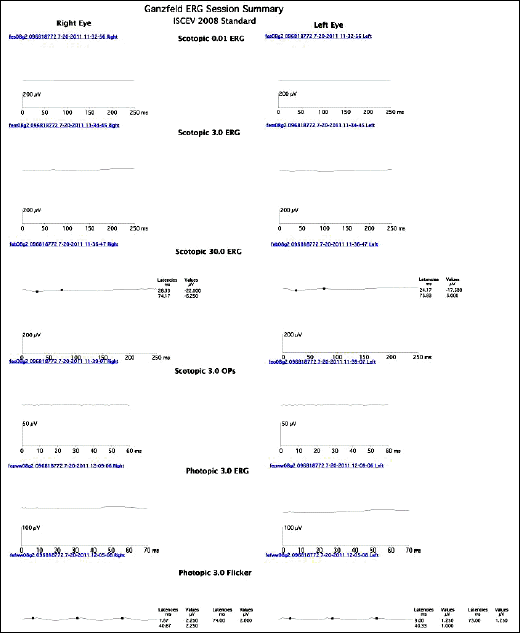
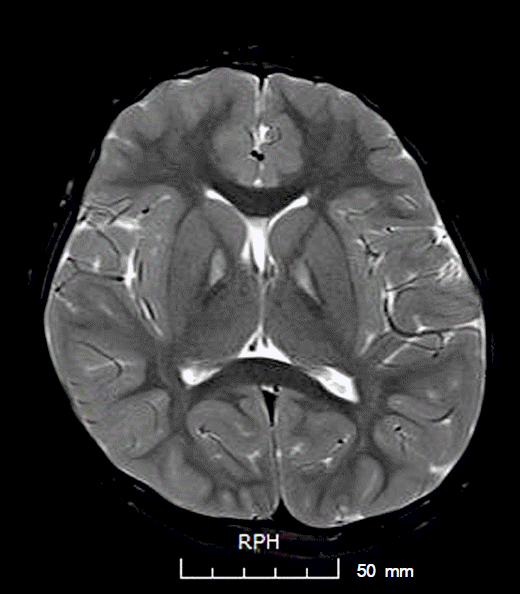
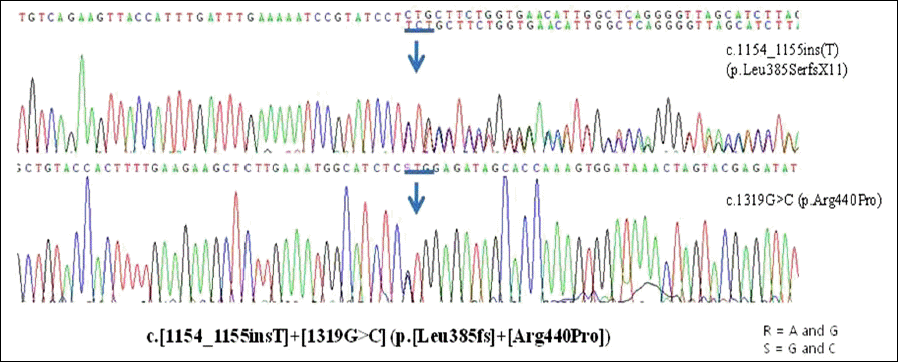
A 6-year-old girl presented with night blindness and developmental delay. Neurologic examination revealed toe gait and dystonia. Ocular examination showed retinal pigmentary change in the entire retina without optic atrophy. Brain magnetic resonance imaging showed iron deposits in the basal ganglia, the so-called eye of the tiger sign. Genetic tests confirmed a mutation in the gene encoding pantothenate kinase 2. Electroretinography demonstrated severe loss of rod and cone responses, prominently reduced in the rod response. The patient was diagnosed with PKAN and pharmacologic treatment started.
References
1. Rock CO, Calder RB, Karim MA, Jackowski S. Pantothenate kinase regulation of the intracellular concentration of coenzyme A. J Biol Chem. 2000; 275:1377–83.

2. Hayflick SJ, Westaway SK, Levinson B, et al. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med. 2003; 348:33–40.

3. Koeppen AH, Dickson AC. Iron in the Hallervorden-Spatz syndrome. Pediatr Neurol. 2001; 25:148–55.

4. Kuo YM, Duncan JL, Westaway SK, et al. Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum Mol Genet. 2005; 14:49–57.
5. Egan RA, Weleber RG, Hogarth P, et al. Neuro-ophthalmologic and electroretinographic findings in pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome). Am J Ophthalmol. 2005; 140:267–74.

6. Gordon N. Pantothenate kinase-associated neurodegeneration (Hallervorden-Spatz syndrome). Eur J Paediatr Neurol. 2002; 6:243–7.

7. Valentino P, Annesi G, Cirò Candiano IC, et al. Genetic heterogeneity in patients with pantothenate kinase-associated neuro-degeneration and classic magnetic resonance imaging eye-of-the-tiger pattern. Mov Disord. 2006; 21:252–4.

8. Zhou B, Westaway SK, Levinson B, et al. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet. 2001; 28:345–9.

9. Johnson MA, Kuo YM, Westaway SK, et al. Mitochondrial localization of human PANK2 and hypotheses of secondary iron accumulation in pantothenate kinase-associated neurodegeneration. Ann N Y Acad Sci. 2004; 1012:282–98.

10. Hayflick SJ, Penzien JM, Michl W, et al. Cranial MRI changes may precede symptoms in Hallervorden-Spatz syndrome. Pediatr Neurol. 2001; 25:166–9.

11. Hayflick SJ. Unraveling the Hallervorden-Spatz syndrome: pantothenate kinase-associated neurodegeneration is the name. Curr Opin Pediatr. 2003; 15:572–7.
12. Feliciani M, Curatolo P. Early clinical and imaging (high-field MRI) diagnosis of Hallervorden-Spatz disease. Neuroradiology. 1994; 36:247–8.

13. Ponka P. Hereditary causes of disturbed iron homeostasis in the central nervous system. Ann N Y Acad Sci. 2004; 1012:267–81.

14. Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet. 2009; 46:73–80.

15. Tripathi RC, Tripathi BJ, Bauserman SC, Park JK. Clinicopathologic correlation and pathogenesis of ocular and central nervous system manifestations in Hallervorden-Spatz syndrome. Acta Neuropathol. 1992; 83:113–9.

16. He X, Hahn P, Iacovelli J, et al. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 2007; 26:649–73.

17. Battistella PA, Midena E, Suppiej A, Carollo C. Optic atrophy as the first symptom in Hallervorden-Spatz syndrome. Childs Nerv Syst. 1998; 14:135–8.

18. Newell FW, Johnson RO 2nd, Huttenlocher PR. Pigmentary degeneration of the retina in the Hallevorden-Spatz syndrome. Am J Ophthalmol. 1979; 88:467–71.
Figure 1.
Fundus photographs show scattered bone-spicule formations, peripheral retinal depigmentation, and the retinal vessel changes in both eyes (A, B).
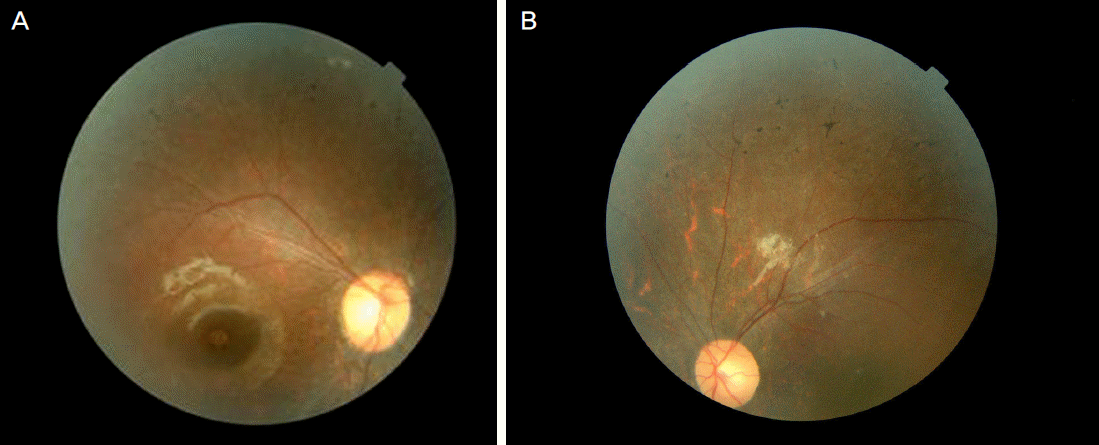
Figure 2.
Electroretinography demonstrated severe loss of rod and cone responses, prominently reduced in rod response.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download