Abstract
Purpose
To evaluate the effect of intravitreal expansile gas (C3F8) with anti-VEGF injection for the treatment of large submacular hemorrhage (SMH) secondary to age-related macular degeneration (ARMD).
Methods
In this report, 18 eyes of 18 patients with large SMH secondary to ARMD were treated with a simultaneous injection of 0.3 cc C3F8 and 0.05 ml anti-VEGF intravitrealy.
Results
The mean age was 64.89 ± 5.68 years and the mean size of SMH was 4.44 ± 1.25 disc diameters (DD). The minimum follow-up period was 12 months (range: 12-17 months). Mean preoperative best corrected visual acuity (BCVA) was 1.72 ± 0.56 log MAR which improved significantly to 1.01 ± 0.68 log MAR at 12 months (p = 0.002). SMH displacement occurred in all eyes. BCVA improved 2 or more lines in 11 eyes (61.1%) and deteriorated in 1 eye (5.6%).
Go to : 
References
1. Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999; 213:97–102.

2. Toth CA, Morse LS, Hjelmeland LM, Landers MB 3rd. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol. 1991; 109:723–9.

3. Lewis H. Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainage. Am J Ophthalmol. 1994; 118:559–68.

4. Ibanez HE, Williams DF, Thomas MA, et al. Surgical management of submacular hemorrhage. A series of 47 consecutive cases. Arch Ophthalmol. 1995; 113:62–9.

5. Lincoff H, Kreissig I. Intravitreal injection of tissue plasminogen activator and gas in subretinal hemorrhage caused by age-related macular degeneration. Retina. 2001; 21:191.

6. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990; 109:33–7.

7. Park KH, Song SJ, Lee WK, et al. The Results of nation-wide registry of age-related macular degeneration in Korea. J Korean Ophthalmol Soc. 2010; 51:516–23.
8. Vander JF, Federman JL, Greven C, et al. Surgical removal of massive subretinal hemorrhage associated with age-related macular degeneration. Ophthalmology. 1991; 98:23–7.

9. Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology. 2004; 111:1993–2006.
10. Hillenkamp J, Surguch V, Framme C, et al. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2010; 248:5–11.

11. van Zeeburg EJ, van Meurs JC. Literature review of recombinant tissue plasminogen activator used for recent-onset submacular hemorrhage displacement in age-related macular degeneration. Ophthalmologica. 2013; 229:1–14.

12. Meyer CH, Scholl HP, Eter N, et al. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008; 86:490–4.

13. Hesse L, Meitinger D, Schmidt J. Little effect of tissue plasminogen activator in subretinal surgery for acute hemorrhage in age-related macular degeneration. Ger J Ophthalmol. 1996; 5:479–83.
14. Hrach CJ, Johnson MW, Hassan AS, et al. Retinal toxicity of commercial intravitreal tissue plasminogen activator solution in cat eyes. Arch Ophthalmol. 2000; 118:659–63.

15. Jung JJ, Cho SW. Retinal toxicity of intravitreal tissue plasminogen activator on submacular hemorrhage. J Korean Ophthalmol Soc. 2009; 50:800–3.

16. Averbukh E, Devenyi RG, Lam WC, Berger AR. Pneumatic displacement of submacular hemorrhage. Ophthalmology. 2000; 107:2118–9.
17. Ahn YS, Lee SJ, Park S. Pneumatic displacement of submacular hemorrhage with intravitreal injection of SF6 gas without tissue plasminogen activator. J Korean Ophthalmol Soc. 2003; 44:2811–5.
18. Johnson MW. Pneumatic displacement of submacular hemorrhage. Curr Opin Ophthalmol. 2000; 11:201–6.

19. Moriarty AP, McAllister IL, Constable IJ. Initial clinical experience with tissue plasminogen activator (tPA) assisted removal of submacular haemorrhage. Eye. 1995; 9((Pt 5)):582–8.

20. Oh SB, Cho WB, Moon JW, Kim HC. Effects and prognostic factors of intravitreal bevacizumab injection on choroidal neovascularization from age-related macular degeneration. J Korean Ophthalmol Soc. 2009; 50:202–10.

21. Cho SW, Bae JH, Song SJ. Anatomical non-responder to intravitreal bevacizumab for neovascular age-related macular degeneration. J Korean Ophthalmol Soc. 2010; 51:1464–70.

22. Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol. 2007; 144:886–92.

23. Kuhli-Hattenbach C, Fischer IB, Schalnus R, Hattenbach LO. Subretinal hemorrhages associated with age-related macular degeneration in patients receiving anticoagulation or antiplatelet therapy. Am J Ophthalmol. 2010; 149:316–21.

24. Hasegawa T, Otani A, Sasahara M, et al. Prognostic factors of vitreous hemorrhage secondary to exudative age-related macular degeneration. Am J Opthalmol. 2010; 149:322–9.

25. Daneshvar H, Kertes PJ, Leonard BC, Peyman GA. Management of submacular hemorrhage with intravitreal sulfur hexafluoride: a pilot study. Can J Ophthalmol. 1999; 34:385–8.
26. Tilanus MA, Vaandrager W, Cuypers MH, et al. Relationship between anticoagulant medication and massive intraocular hemorrhage in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2000; 238:482–5.

27. Kernt M, Neubauer AS, Kampik A. Intravitreal bevacizumab (Avastin) treatment is safe in terms of intraocular and blood pressure. Acta Ophthalmol Scand. 2007; 85:119–20.
28. Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 2008; 146:930–4.

Go to : 
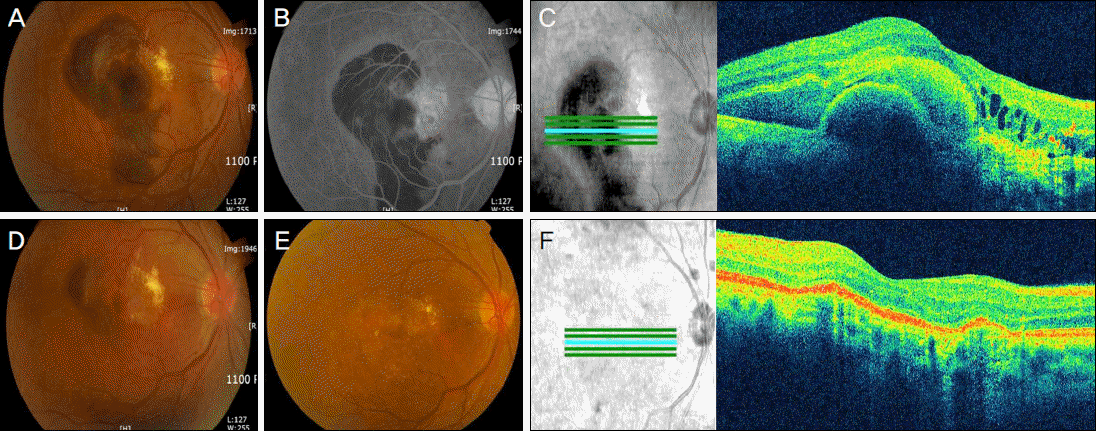 | Figure 1.Color photograph (A), fluorescein angiography (B) and optical coherence tomography (C) of case 1 before treatment. Postoperative 3 days after intravitreal C3F8 and anti-VEGF injection (D). On 12 months, color photograph (E) and optical coherence tomography (F). |
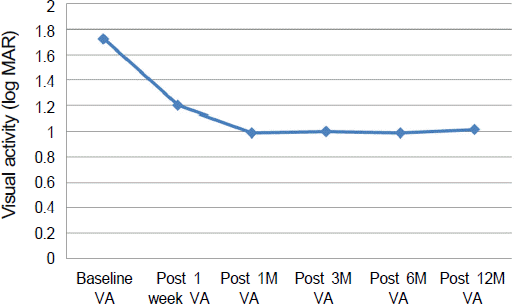 | Figure 2.Mean visual acuity changes from baseline through 12 months. *VA = visual acuity; †M = month. |
Table 1.
Patient's characteristics
Table 2.
Changes in visual acuity
| Visual acuity (mean ± SD, log MAR) | p-value (difference from baseline) | p-value (difference from Prior VA) | |
|---|---|---|---|
| Prior to injection | 1.72 ± 0.56 | ||
| 1 week after injection | 1.20 ± 0.41 | 0.000* | 0.000 |
| 1 month after injection | 0.99 ± 0.49 | 0.000* | 0.011 |
| 3 months after injection | 1.00 ± 0.57 | 0.001* | 0.286 |
| 6 months after injection | 0.99 ± 0.65 | 0.002* | 0.480 |
| 12 months after injection | 1.02 ± 0.67 | 0.002* | 0.914 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download