Abstract
Purpose
To investigate the clinical effect of micro-multiporous e-PTFE insertion for severe recurrent pterygium with symblepharon.
Methods
The present study included a total of 13 cases of recurrent pterygium associated with symblepharon, motility restriction and diplopia which had undergone micro-multiporous e-PTFE insertion after pterygium excision, 0.02% mitomycin C application, human amniotic membrane transplantation (AMT) and/or conjunctivo-limbal autograft (CLAU) between September 2010 and February 2011. One month after surgery, the inserted e-PTFE was removed. Recurrence of pterygium and symblepharon, motility restriction, diplopia and injection of ocular surface were evaluated for 11.92 ± 1.32 months of mean follow-up period.
Results
Pterygial recurrence was not observed in 12 out of 13 eyes, and the 1 eye which recurred showed conjunctival recurrence. No postoperative symblepharon recurrence was observed in any of the 13 eyes. Diplopia and motility restriction disappeared in 11 out of 13 eyes, and were improved in the other 2 eyes. VAS (Visual Analogue Scale) injection scores in the wound site decreased after surgery in all patients.
Go to : 
References
1. Frucht-Pery J, Ilsar M, Hemo I. Single dosage of mitomycin C for prevention of recurrent pterygium: preliminary report. Cornea. 1994; 13:411–3.

2. Lam DS, Wong AK, Fan DS, et al. Intraoperative mitomycin C to prevent recurrence of pterygium after excision: a 30-month follow-up study. Ophthalmology. 1998; 105:901–4.
3. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997; 104:974–85.

4. Shimazaki J, Kosaka K, Shimmura S, Tsubota K. Amniotic membrane transplantation with conjunctival autograft for recurrent pterygium. Ophthalmology. 2003; 110:119–24.

5. Shimazaki J, Shinozaki N, Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol. 1998; 82:235–40.

7. Barraquer MJ. [Localized discontinuity of the precorneal lacrimal film. Etiology of Fuchs' marginal corneal ulcers, of progression of pterygium and of certain corneal necroses in the neighborhood of keratoprostheses and keratoplasties]. Ophthalmologica. 1965; 150:111–22.
8. Hirst LW. Recurrent pterygium surgery using pterygium extended removal followed by extended conjunctival transplant: recurrence rate and cosmesis. Ophthalmology. 2009; 116:1278–86.

9. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001; 108:449–60.

10. Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005; 24:141–50.

11. Küçükerdönmez C, Akova YA, Altinörs DD. Comparison of conjunctival autograft with amniotic membrane transplantation for pterygium surgery: surgical and cosmetic outcome. Cornea. 2007; 26:407–13.
12. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997; 115:1235–40.

13. Kenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985; 92:1461–70.

14. Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002; 109:1752–5.

15. Luanratanakorn P, Ratanapakorn T, Suwan-Apichon O, Chuck RS. Randomised controlled study of conjunctival autograft versus amniotic membrane graft in pterygium excision. Br J Ophthalmol. 2006; 90:1476–80.

16. Jenkins SD, Klamer TW, Parteka JJ, Condon RE. A comparison of prosthetic materials used to repair abdominal wall defects. Surgery. 1983; 94:392–8.
17. Karesh JW. Polytetrafluoroethylene as a graft material in ophthalmic plastic and reconstructive surgery. An experimental and clinical study. Ophthal Plast Reconstr Surg. 1987; 3:179–85.
18. Hanel KC, McCabe C, Abbott WM, et al. Current PTFE grafts: a biomechanical, scanning electron, and light microscopic evaluation. Ann Surg. 1982; 195:456–63.
19. Kim SJ, Khwarg SI, Choung HK. Expanded polytetrafluoroethylene as a wrapping material for porous polyethylene orbital implant. J Korean Ophthalmol Soc. 2007; 48:117–24.
20. Lee JH, Ham DI, Lee JH, et al. Experimental keratoprosthesis using expanded PTFE(Gore-Tex) as a supporting skirt. J Korean Ophthalmol Soc. 1992; 33:555–63.
21. Kim DM, Hyung SM. Fibrous capsule surrounding silicone encircling band and core texTM surgical membrane : a histological study. J Korean Ophthalmol Soc. 1991; 32:22–8.
22. Yu C, Tang X, Wan J. [A clinical study of polytetrafluoroethylene (polytef) as a substitute for conjunctiva]. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1996; 12:447–8.
23. Schmidt T, Kohler W. [Fornix reconstruction in ocular pemphigoid with Goretex surgical membrane]. Ophthalmologe. 1997; 94:321–3.
24. Liu J, Fu Y, Xu Y, Tseng SC. New grading system to improve the surgical outcome of multirecurrent pterygia. Arch Ophthalmol. 2012; 130:39–49.

25. Kao LY. Polytetrafluoroethylene as a wrapping material for a hydroxyapatite orbital implant. Ophthal Plast Reconstr Surg. 2000; 16:286–8.

26. Bradley TR, Hodgson GS, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978; 97:517–22.

27. Bradley JC, Yang W, Bradley RH, et al. The science of pterygia. Br J Ophthalmol. 2010; 94:815–20.

29. Yao YF, Qiu WY, Zhang YM, Tseng SC. Mitomycin C, amniotic membrane transplantation and limbal conjunctival autograft for treating multirecurrent pterygia with symblepharon and motility restriction. Graefes Arch Clin Exp Ophthalmol. 2006; 244:232–6.

30. Yamamoto T, Varani J, Soong HK, Lichter PR. Effects of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival fibroblasts. Ophthalmology. 1990; 97:1204–10.

31. Hayasaka S, Noda S, Yamamoto Y, Setogawa T. Postoperative instillation of low-dose mitomycin C in the treatment of primary pterygium. Am J Ophthalmol. 1988; 106:715–8.

32. Güler M, Sobaci G, Ilker S, et al. Limbal-conjunctival autograft transplantation in cases with recurrent pterygium. Acta Ophthalmol (Copenh). 1994; 72:721–6.

33. Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004; 88:911–4.

34. Koranyi G, Seregard S, Kopp ED. The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand. 2005; 83:298–301.

35. Kourembanas S, Hannan RL, Faller DV. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990; 86:670–4.

36. Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991; 88:1054–7.

37. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992; 359:843–5.

38. Knighton DR, Hunt TK, Scheuenstuhl H, et al. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983; 221:1283–5.

39. Falanga V, Qian SW, Danielpour D, et al. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. J Invest Dermatol. 1991; 97:634–7.
40. Falanga V, Kirsner RS. Low oxygen stimulates proliferation of fibroblasts seeded as single cells. J Cell Physiol. 1993; 154:506–10.

Go to : 
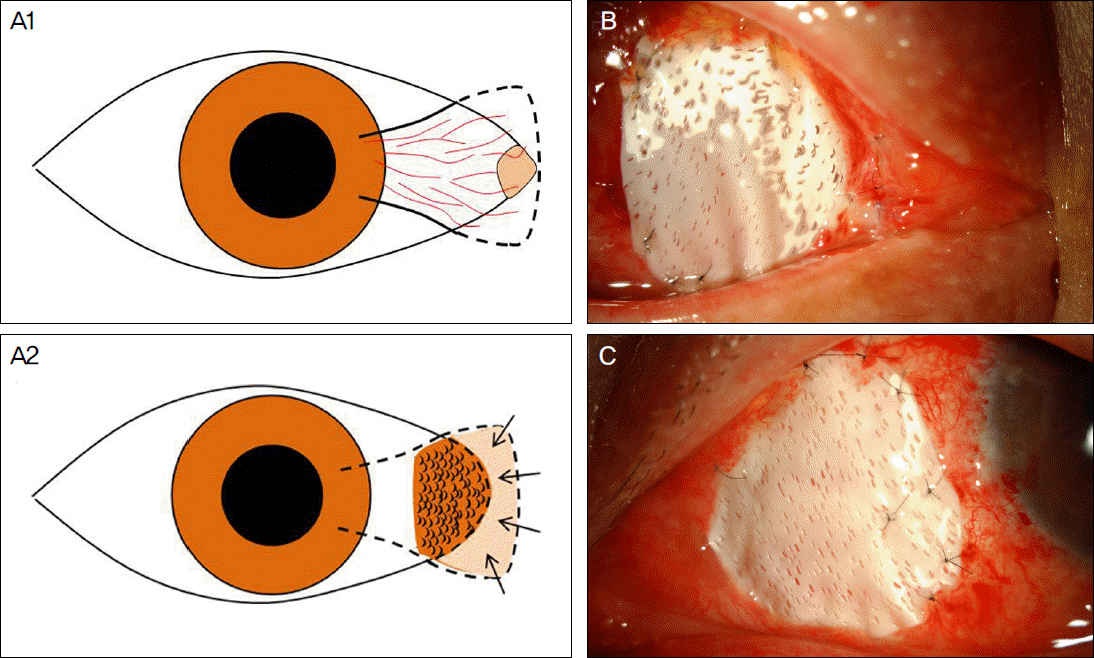 | Figure 1.(A) Diagrammatic representation of patient with recurrent pterygium, preoperative (A1) and postoperative (A2). (A2) M icro-multiporous e-PTFE is inserted and well-fitted with the gap sealed between the conjunctiva and post-excised pterygium wound site. Note that there is no micropore on e-PTFE, especially under the buried site (arrows) to prevent fibrovascular tissue from growing through. (B, C) E-PTFE is inserted under the remained nasal conjunctiva, with micopores only at the area exposed to air. |
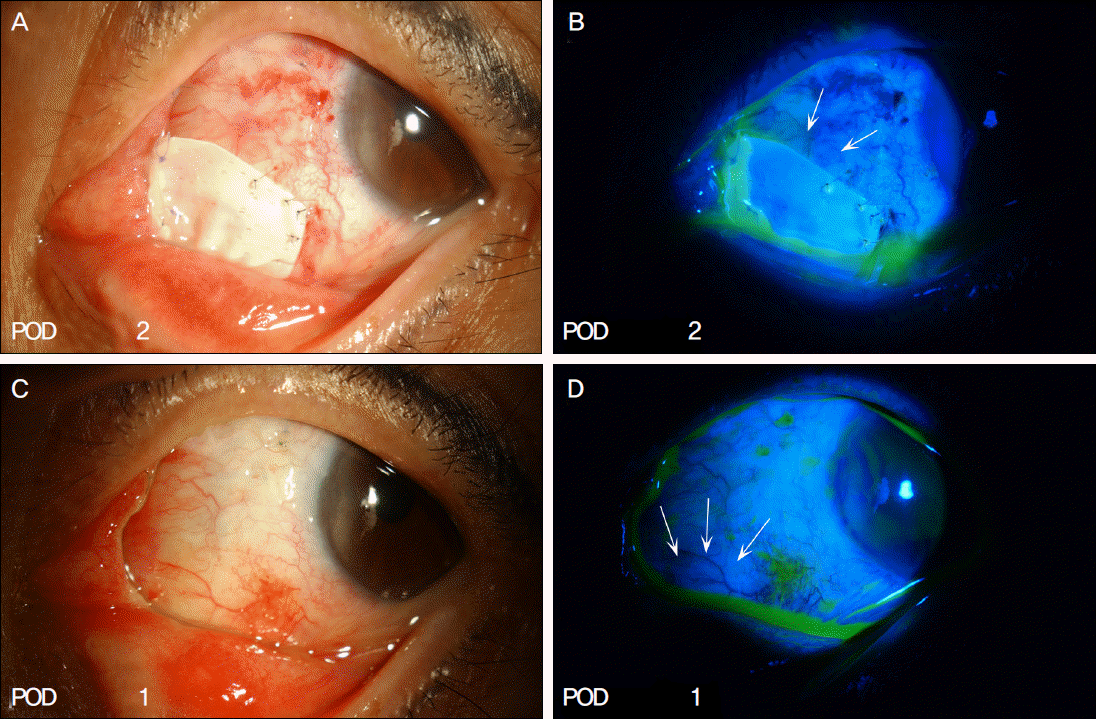 | Figure 2.Postoperative appearances of epithelialization after fluorescein staining (Case 7). (A, B) At 2 weeks after the surgery, epithelization is observed on exposed permanent AM graft site (arrows). (C, D) Epithelization is observed when e-PTFE is removed 1 month after the surgery at the previously multiporous e-PTFE-implanted site (arrows). |
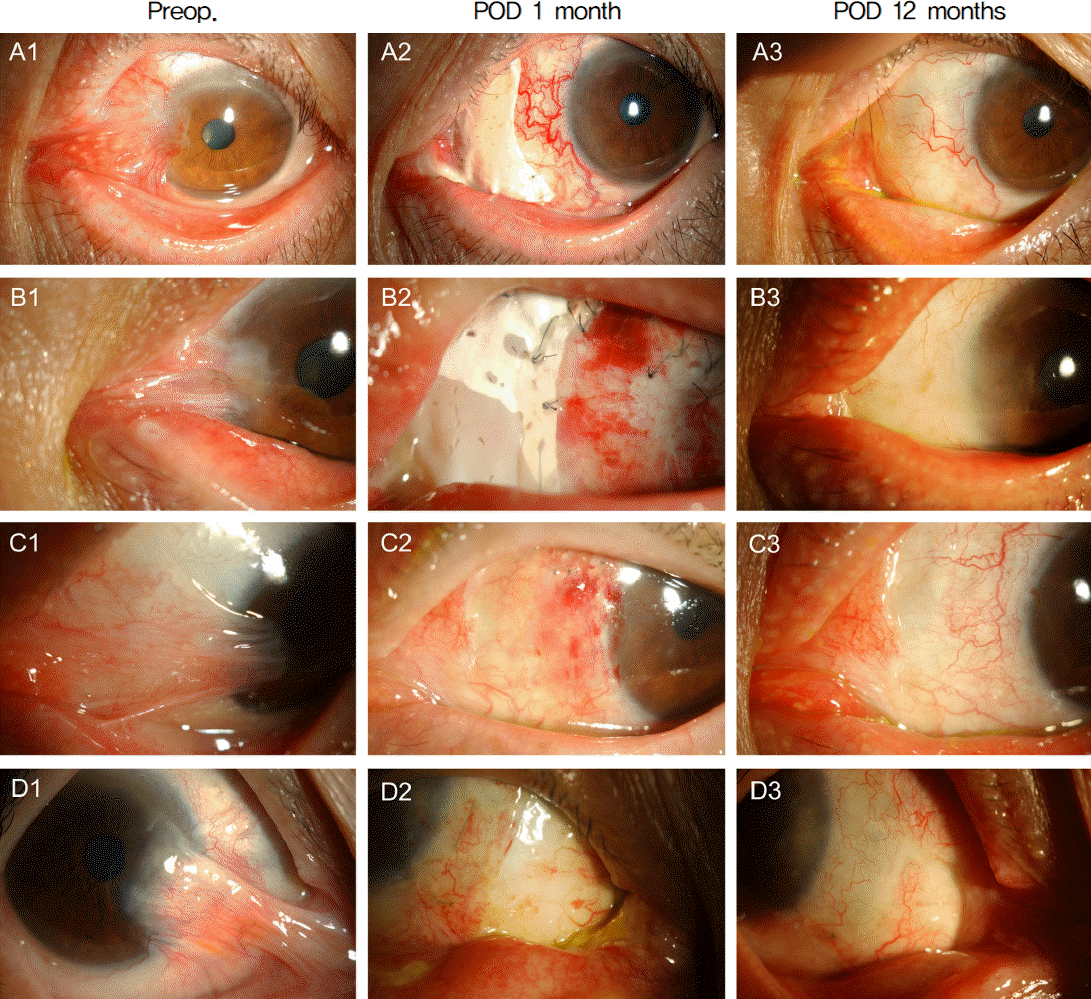 | Figure 3.Preoperative and postoperative appearances of case 1 (A), case 2 (B), case 3 (C), case 6 (D). Preoperatively, symblepharon of the eyelid and the nasal caruncle adhered to the cornea are noted (A1-D1). M icro-multiporous e-PTFE was removed 1 month after the surgery (A2, B2: just before removal; C2, D2: just after removal). Postoperatively, fibrovascular tissue was completely removed, and recurrence was not noted 12 months after the surgery (A3-D3). |
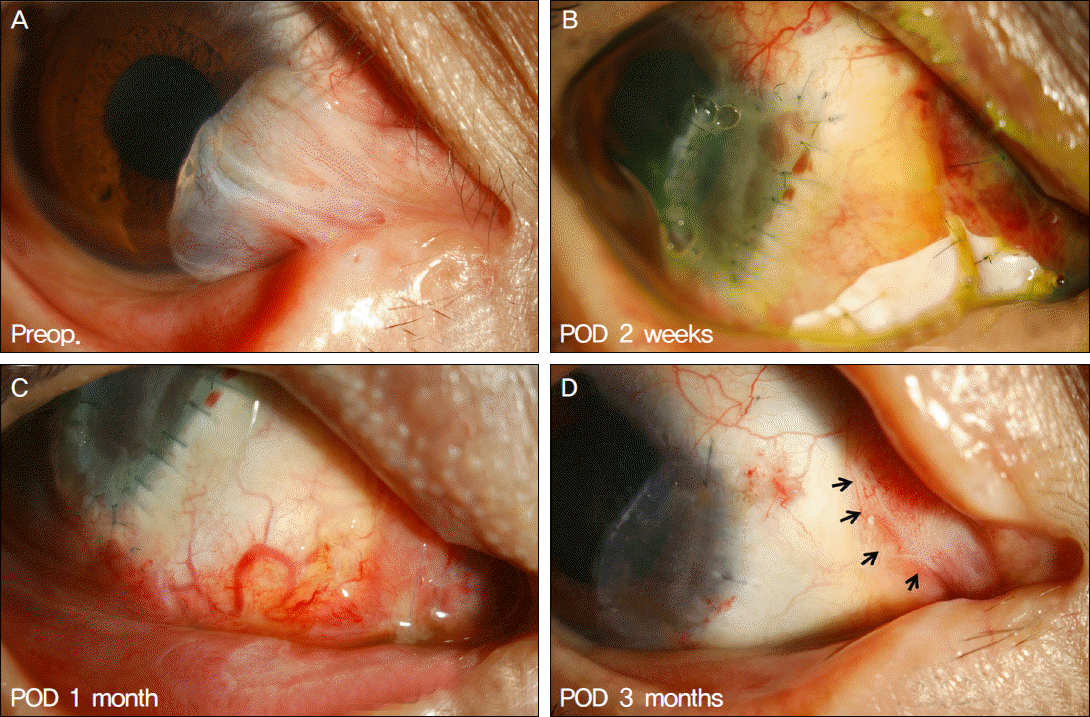 | Figure 4.Postoperative pterygial recurrence case (Case 8). (C) Severe injection is noted at the pterygium-excised site 1 month after the surgery. (D) Fibrovascular tissue adhesion is noted (arrows) limited to the excised area, not adjacent to the limbus. |
Table 1.
Summary of clinical data
Table 2.
Clinical grading system for pterygium morphology
Table 3.
Clinical grading system for pterygium recurrence




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download