Abstract
Purpose
Sildenafil citrate was developed to treat angina, but was found to also inhibit PDE in the corpus callosum and is now widely used to treat impotence. We report a case of enlargement of the inferior rectus muscle after sildenafil citrate ingestion.
Case summary
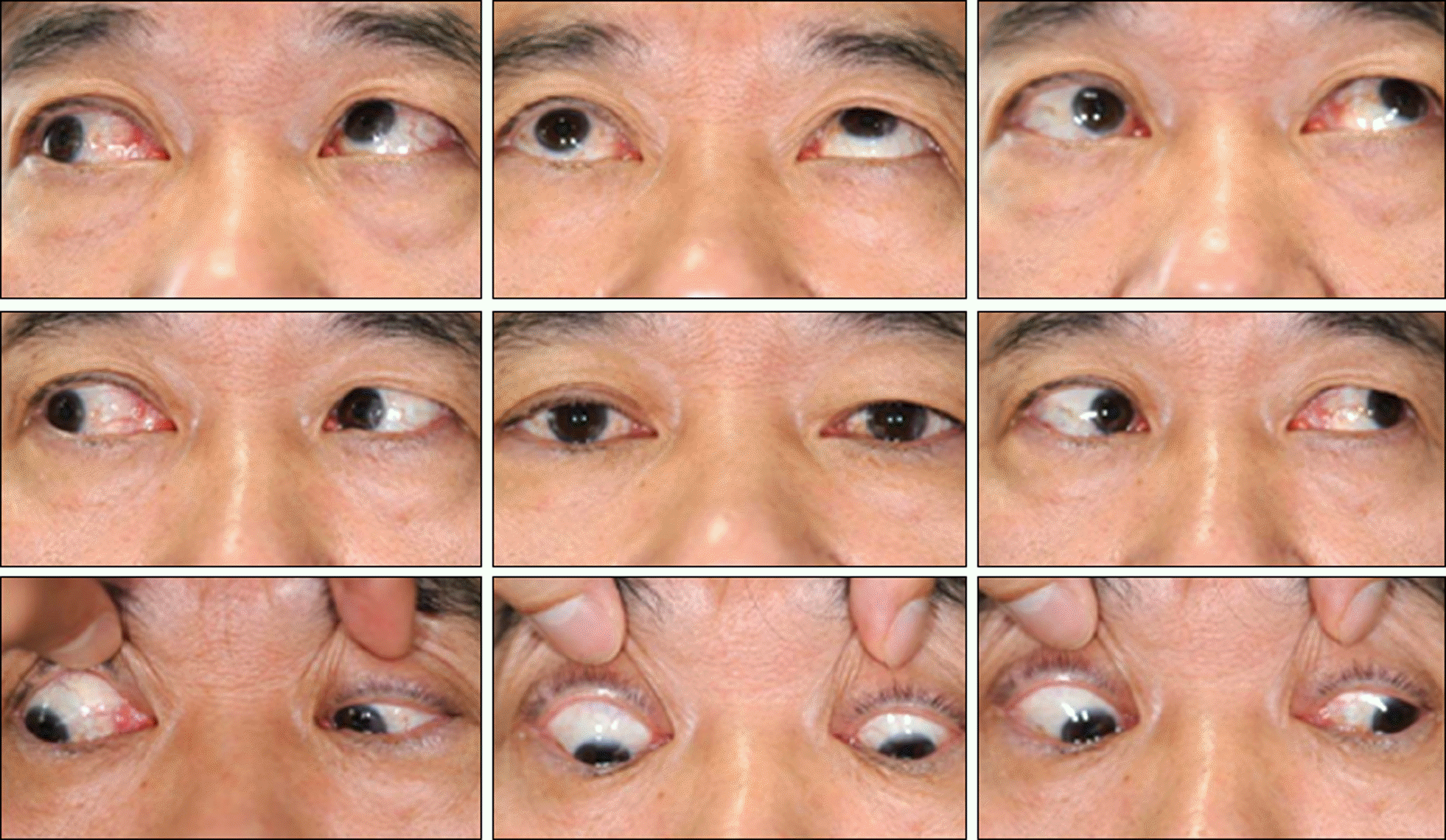
A 58-year-old male presented with binocular diplopia which started 2 weeks prior. He had no underlying disease. The patient had taken sildenafil citrate on 3 different occasions 2 weeks prior, and 2 days after his first ingestion, his right conjunctiva was injected and binocular diplopia started. On the first visit, he showed 16 PD hypotropia of the right eye with limitation of motion at upgaze. The MRI scan showed enlargement and enhancement of the inferior rectus muscle, and 50 mg of prednisone was prescribed. The amount of hypotropia decreased to 8 PD 2 weeks later.
References
2. Sivaswamy L, Vanstavern GP. Ischemic optic neuropathy in a child. Pediatr Neurol. 2007; 37:371–2.

3. Tripathi A, O'Donnell NP. Branch retinal artery occlusion; another complication of sildenafil. Br J Ophthalmol. 2000; 84:934–5.

4. Donahue SP, Taylor RJ. Pupil-sparing third nerve palsy associated with sildenafil citrate (Viagra). Am J Ophthalmol. 1998; 126:476–7.

5. Jang YS, Ahn GS, Kim SD. Retinal hemorrhage associated with viagra (sildenafil citrate). J Korean Ophthalmol Soc. 2002; 43:1340–4.
6. Fraunfelder FW, Pomeranz HD, Egan RA. Nonarteritic anterior ischemic optic neuropathy and sildenafil. Arch Ophthalmol. 2006; 124:733–4.

7. Laties AM. Vision disorders and phosphodiesterase type 5 inhibitors: a review of the evidence to date. Drug Saf. 2009; 32:1–18.
8. Lee WJ, Seong M. Bilateral simultaneous acute angle closure glaucoma following sexual intercourse aided by sildenafil citrate. J Korean Ophthalmol Soc. 2011; 52:1123–7.

9. Lee JS, Oum BS, Choi CH, Kim HJ. Clinical study of the idiopathic orbital myositis. J Korean Ophthalmol Soc. 1999; 40:1109–15.
10. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30:239–45.

11. Kim CH, Lim JY, Ahn JH, Jang JW. A case of idiopathic orbital myositis involving all extraocular muscles of both eyes. J Korean Ophthalmol Soc. 2001; 42:1615–20.
12. Mombaerts I, Koornneef L. Current status in the treatment of orbital myositis. Ophthalmology. 1997; 104:402–8.

13. Weinstein GS, Dresner SC, Slamovits TL, Kennerdell JS. Acute and subacute orbital myositis. Am J Ophthalmol. 1983; 96:209–17.

Figure 2.
Axial (A), coronal (B), sagittal (C, D) M RI images show enlargement and enhancement of the inferior rectus muscle of the right eye. There is no enlargement of the extraocular muscles of the left eye.
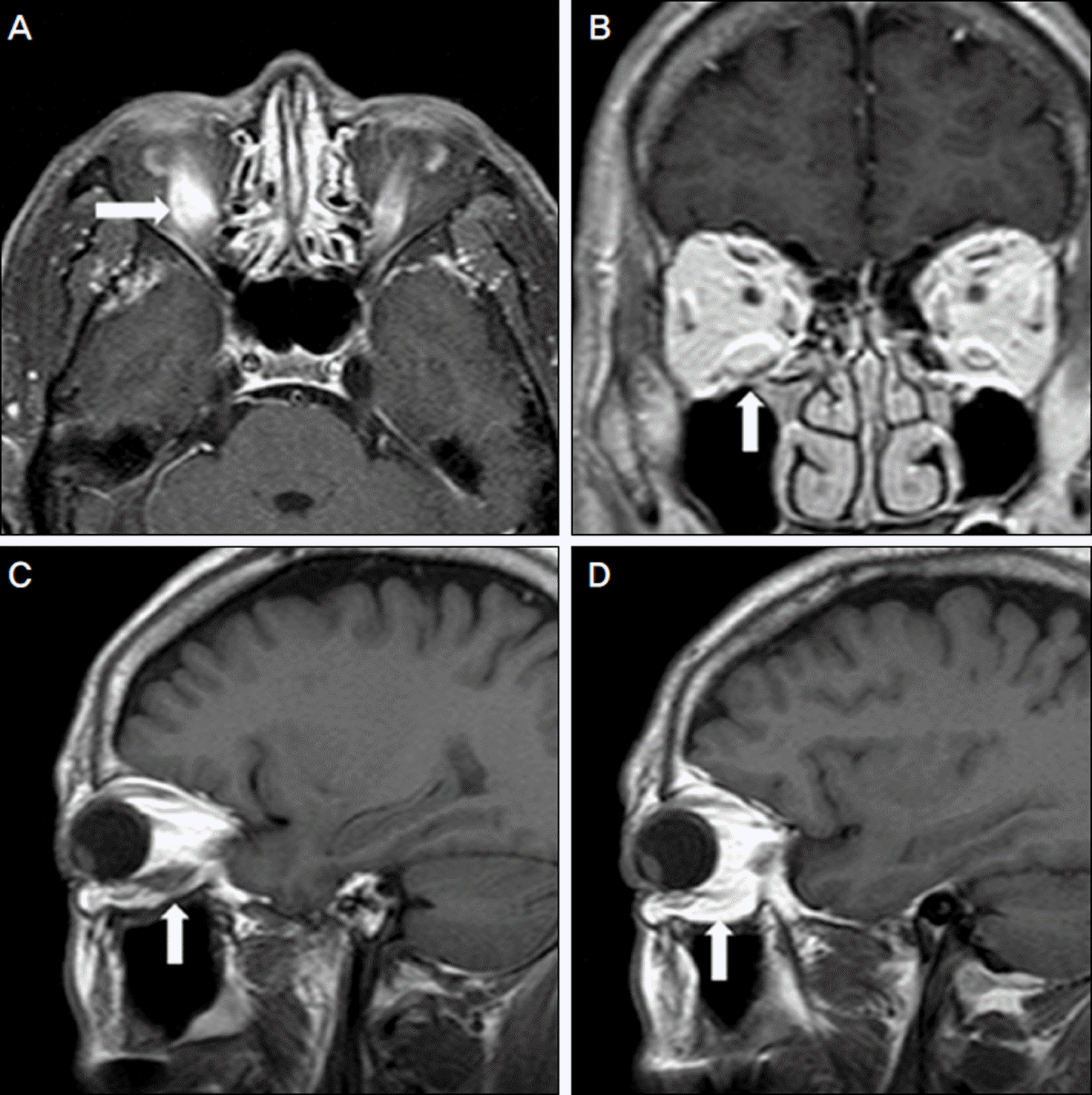
Table 1.
The Naranjo adverse drug reaction probability scale




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download