Abstract
Purpose
To evaluate the changes of anterior chamber parameters and intraocular pressure (IOP) with Pentacam® after intravitreal injection.
Methods
A total of 76 eyes of 76 patients received an intravitreal injection of either triamcinolone acetonide (TA) or bevacizumab. Twelve patients were treated with an intravitreal injection of TA 0.1 ml, 16 patients were treated with an intravitreal injection of TA 0.05 ml, while the remaining 48 patients received a bevacizumab 0.05 ml injection. All patients underwent anterior chamber depth, anterior chamber angle, and anterior chamber volume evaluation with Pentacam® before and 5 minutes after injection. Additionally, IOP measurements were taken 5 minutes before and 5 minutes, 30 minutes, 1 hour and 1 day after injection.
Results
Anterior chamber depth, anterior chamber angle, anterior chamber volume, and IOP changes in patients receiving TA 0.1 ml were 0.4 ± 0.11 mm, 10.2 ± 4.1°, 33.7 ± 5.9 mm3 and 18.8 ± 12.1 mm Hg, respectively. Anterior chamber depth, anterior chamber angle, anterior chamber volume, and IOP changes in patients receiving TA 0.05 ml were -0.01 ± 0.05 mm, 2.4 ± 3.2°, 5.8 ± 9.5 mm3 and 4.8 ± 7.4 mm Hg, respectively. Anterior chamber depth, anterior chamber angle, anterior chamber volume, and IOP changes in patients receiving bevacizumab were 0.28 ± 0.99 mm, 0.8 ± 4.0°, 7.1 ± 9.6 mm3 and 5.4 ± 6.3 mm Hg, respectively. There was a significant difference between TA 0.1 ml and 0.05 ml. However, there was no significant difference between TA 0.05 ml and bevacizumab 0.05 ml.
Conclusions
Because of similar anterior chamber parameters changes after 0.05 ml intravitreal injection with TA or bevacizumab, early period IOP increases due to intravitreal volume expansion. Intravitreal 0.05 ml injections do not require any other procedures for controlling IOP 30 minutes after injection.
References
1. Kim TH, Moon YS, Chin HS. Change of residual period and clearance rate of intravitreal triamcinolone according to initial injection dosage. J Korean Ophthalmol Soc. 2005; 46:1569–74.
2. Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol. 2007; 52:503–22.

3. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intra-vitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.

4. Roth DB, Realini T, Feuer WJ. . Short-term complications of intravitreal injection of triamcinolone acetonide. Retina. 2008; 28:66–70.

5. Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal tri-amcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004; 138:740–3.

6. Wingate RJ, Beaumont PE. Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthalmol. 1999; 27:431–2.
7. Ozkiriş A, Erkiliç K. Complications of intravitreal injection of tri-amcinolone acetonide. Can J Ophthalmol. 2005; 40:63–8.
8. Yang YH, Kim KR, Yang SW, Yim HB. The effect of intravitreal triamcinolone acetonide on intraocular pressure. J Korean Ophthalmol Soc. 2004; 45:1081–5.
9. Bakri SJ, Beer PM. The effect of intravitreal triamcinolone aceto-nide on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003; 34:386–90.

10. Park HY, Yi K, Kim HK. Intraocular pressure elevation after intra-vitreal triamcinolone acetonide injection. Korean J Ophthalmol. 2005; 19:122–7.

11. Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin). Retina. 2007; 27:1044–7.

12. Hattenbach LO, Klais C, Koch FH, Gümbel HO. Intravitreous in-jection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001; 108:1485–92.

13. Kerimoglu H, Ozturk BT, Bozkurt B. . Does lens status affect the course of early intraocular pressure and anterior chamber changes after intravitreal injection? Acta Ophthalmol. 2011; 89:138–42.

14. Höhn F, Mirshahi A. Impact of injection techniques on intraocular pressure (IOP) increase after intravitreal ranibizumab application. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1371–5.

15. Singh IP, Ahmad SI, Yeh D. . Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2004; 138:286–7.

16. Park SY, Chol KS. Intraocular pressure elevation after intravitreal triamcinolone acetonide of different volumes: Comparing 0.1 ml vs 0.05 ml. J Korean Ophthalmol Soc. 2008; 49:589–94.

17. Hariprasad SM, Shah GK, Blinder KJ. Short-term intraocular pres-sure trends following intravitreal pegaptanib (Macugen) injection. Am J Ophthalmol. 2006; 141:200–1.

18. Lee EW, Hariprasad SM, Mieler WF. . Short-term intraocular pressure trends after intravitreal triamcinolone injection. Am J Ophthalmol. 2007; 143:365–7.

19. Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin). Retina. 2007; 27:1044–7.

20. Hollands H, Wong J, Bruen R. . Short-term intraocular pres-sure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007; 42:807–11.

21. Hollands H, Wong J, Bruen R. . Short-term intraocular pres-sure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007; 42:807–11.

22. Sharei V, Höhn F, Köhler T. . Course of intraocular pressure af-ter intravitreal injection of 0.05 mL ranibizumab (Lucentis). Eur J Ophthalmol. 2010; 20:174–9.

23. Shankar H, Taranath D, Santhirathelagan CT, Pesudovs K. Anterior segment biometry with the Pentacam: comprehensive as-sessment of repeatability of automated measurements. J Cataract Refract Surg. 2008; 34:103–13.

Figure 1.
Preop (A) and postop (B) Pentacam® images of intravitreal triamcinolon 0.05 ml injection patient.
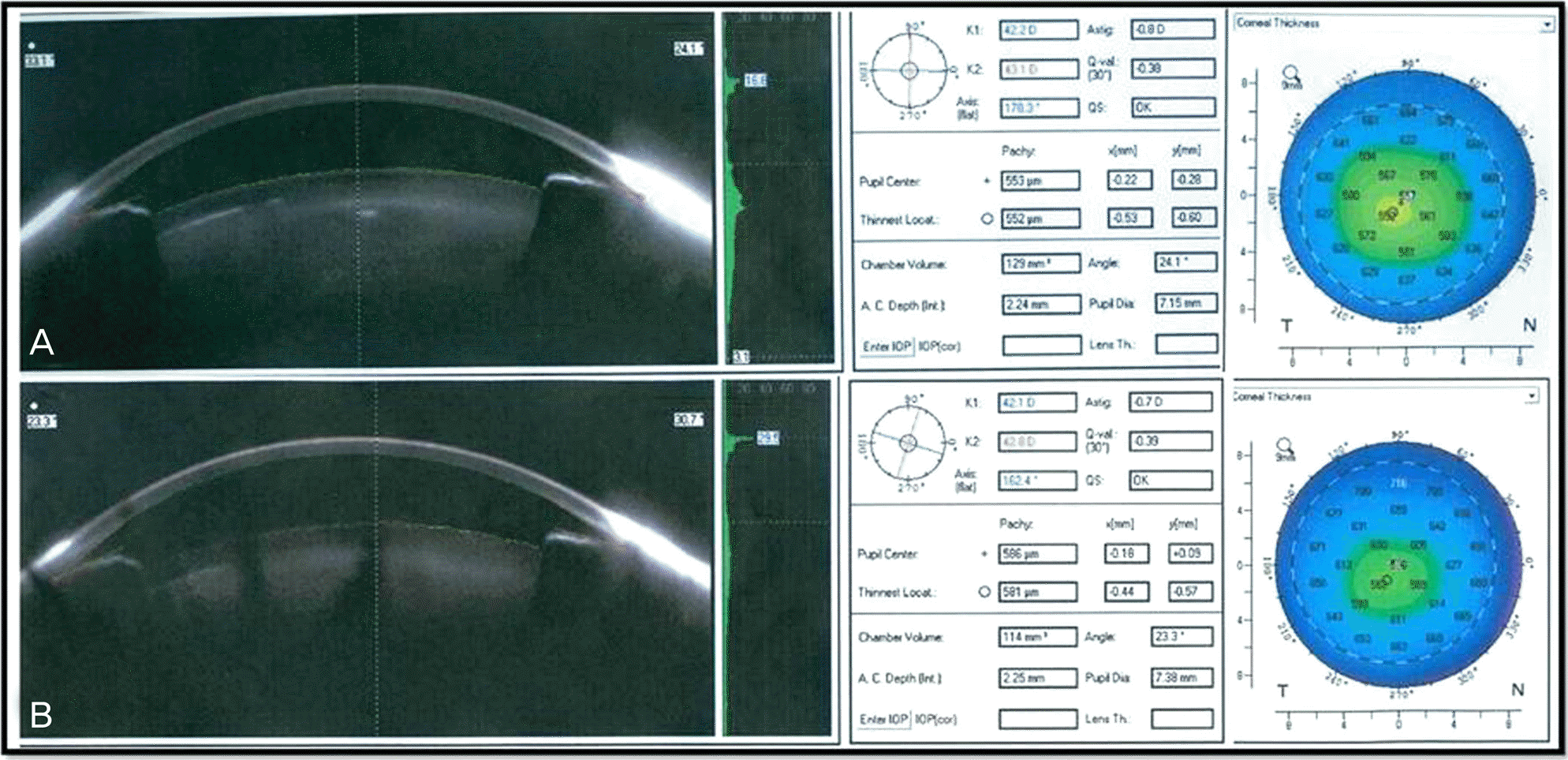
Figure 2.
Preop (A) and postop (B) Pentacam® images of intravitreal triamcinolone 0.1 ml injection patient.
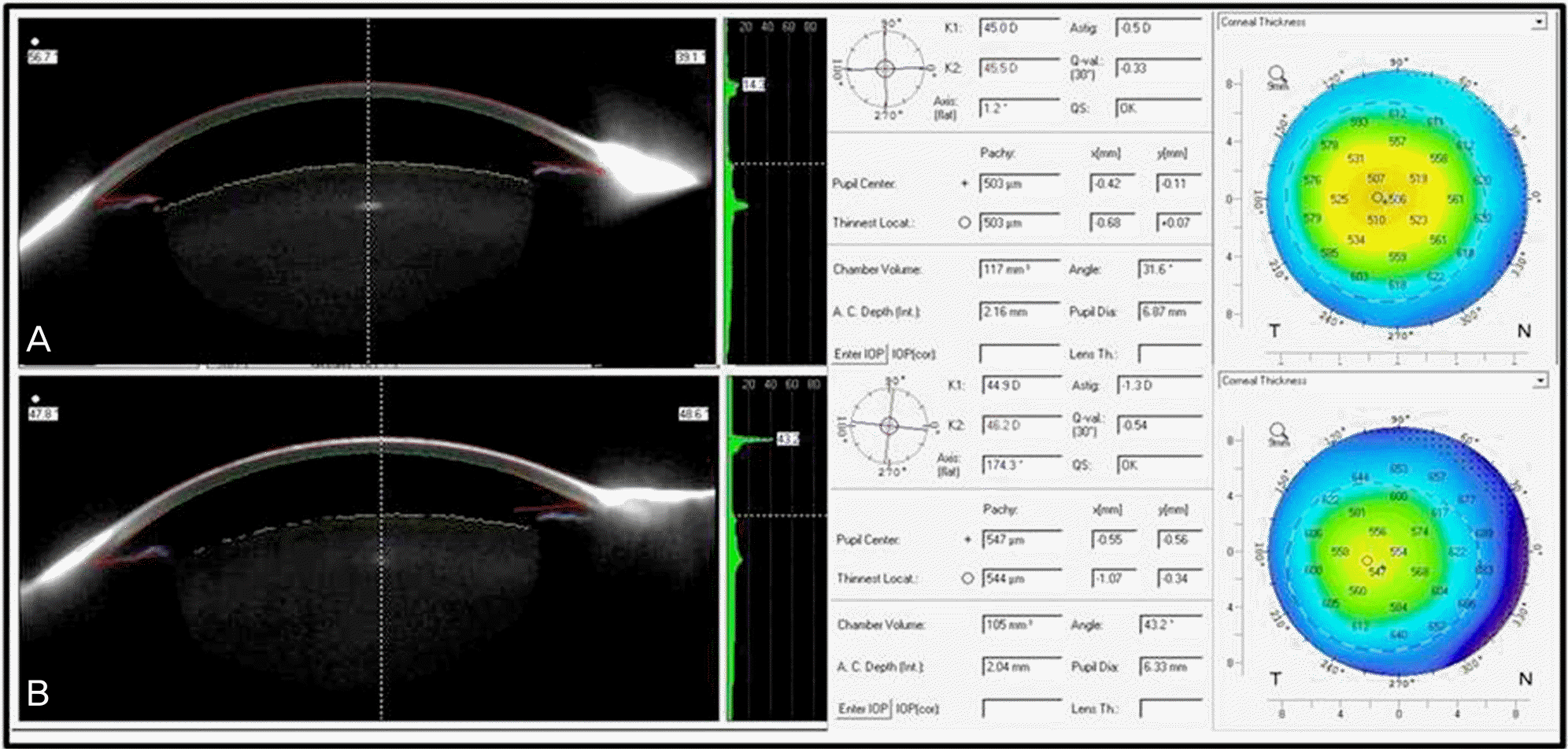
Figure 3.
IOP changes in IVTA patients and IVB or IVR patients. Preop (I) = immediate before injection; Postop (I) = immediate after injection; Dash line = IVB patients; Continuous line = IVTA 0.05 ml patients.
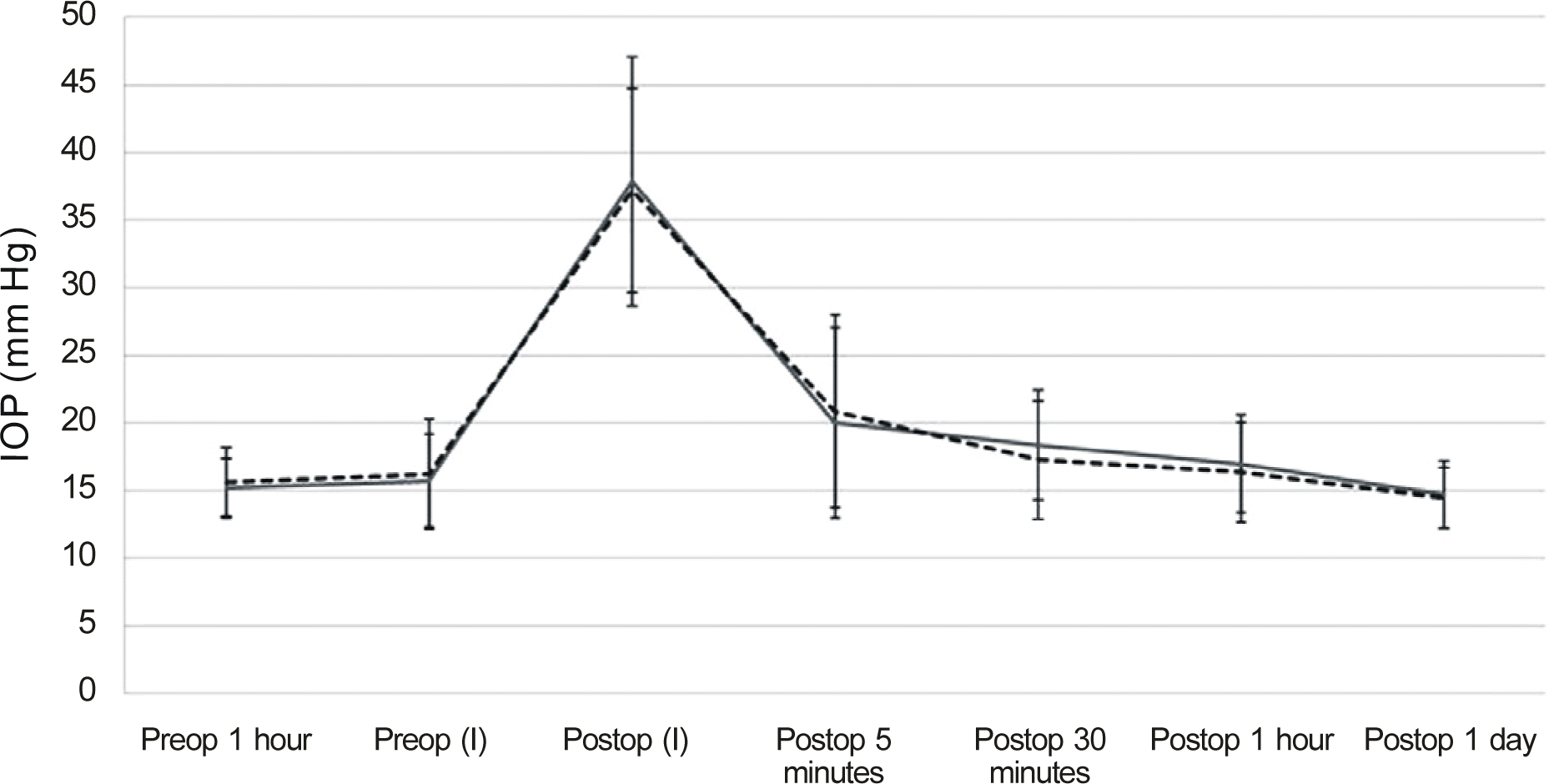
Table 1.
Patients demographics
| Total patients | IVTA 0.1 ml patients | IVTA 0.05 ml patients | IVB patients | p-value* | |
|---|---|---|---|---|---|
| Number of eyes | 76 | 12 | 16 | 48 | |
| Sex | 0.201 | ||||
| Male | 38 | 10 | 6 | 22 | |
| Female | 38 | 2 | 10 | 26 | |
| Age (years) | 55.5 ± 11.2 | 57.6 ± 11.5 | 52.9 ± 10.2 | 56.2 ± 13.7 | 0.786 |
| Laterality | 0.874 | ||||
| OD | 62 | 10 | 12 | 40 | |
| OS | 14 | 2 | 4 | 8 | |
| Diagnosis | |||||
| AMD | 22 | 0 | 0 | 22 | |
| DMR with ME | 40 | 10 | 14 | 16 | |
| BRVO with ME | 9 | 0 | 1 | 8 | |
| CRVO with ME | 5 | 2 | 1 | 2 | |
| Basal IOP (mm Hg) | 15.6 ± 2.2 | 16.3 ± 1.5 | 15.3 ± 2.1 | 15.7 ± 2.6 | 0.708 |
AMD = age related macular degeneration; DMR with ME = diabetic retinopathy with macular edema; BRVO with ME = branch retinal vein occlusion with macular edema; CRVO with ME = central retinal vein occlusion with macular edema; Basal IOP = intraocular pressure of 1 hour before injection; IVTA = intravitreal triamcinolone acetonide injection; IVB = intravitreal bevacizumab injection.
Table 2.
Changes of anterior chamber parameters
|
Total patients |
IVTA 0.1 ml patients |
IVTA 0.05 ml patients |
IVB patients |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre-injection | Post-injection | Pre-injection | Post-injection | Pre-injection | Post-injection | Pre-injection | Post-injection | |
| Corneal thickness | 535.3 ± 28.1 | 566.9 ± 30.1 | 542.2 ± 18.8 | 578.2 ± 13.6 | 535.3 ± 19.8 | 563.4 ± 23.9 | 535.7 ± 31.5 | 569.1 ± 32.9 |
| p-value* | <0.001 | 0.035 | <0.001 | <0.001 | ||||
| ACD | 2.8 ± 0.5 | 2.6 ± 0.6 | 2.7 ± 0.2 | 2.3 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.3 | 2.9 ± 0.8 | 2.7 ± 0.8 |
| p-value* | 0.022 | 0.013 | 0.803 | 0.200 | ||||
| ACV | 147.8 ± 30.1 | 137.3 ± 31.4 | 158.8 ± 23.4 | 125.2 ± 25.1 | 136.5 ± 15.6 | 130.6 ± 11.8 | 146.9 ± 34.3 | 128.5 ± 50.0 |
| p-value* | 0.001 | 0.001 | 0.131 | 0.145 | ||||
| ACA | 29.2 ± 6.3 | 27.0 ± 6.8 | 33.6 ± 7.1 | 23.4 ± 4.8 | 30.3 ± 2.3 | 27.9 ± 2.6 | 28.1 ± 6.4 | 27.3 ± 7.7 |
| p-value* | 0.009 | 0.041 | 0.067 | 0.322 | ||||
Table 3.
Comparison of changes at 5 minutes after intravitreal injection
| IVTA 0.1 ml patients (n = 12) | IVTA 0.05 ml patients (n = 16) | p-valuev* | |
|---|---|---|---|
| △ Corneal Thickness (Pre-Post) | -36.0 ± 10.4 | -28.1 ± 11.1 | 0.201 |
| △ ACD (Pre-post) | 0.4 ± 0.11 | -0.01 ± 0.05 | <0.001 |
| △ ACV (Pre-post) | 33.7 ± 5.9 | 5.8 ± 9.5 | <0.001 |
| △ ACA (Pre-Post) | 10.2 ± 4.1 | 2.4 ± 3.2 | 0.002 |
| △ IOP (I) | 36.8 ± 10.2 | 22.1 ± 8.6 | 0.022 |
| △ IOP (5) | 18.8 ± 12.1 | 4.8 ± 7.4 | 0.021 |
Table 4.
Comparison of changes at 5 minutes after intravitreal injection
| IVTA 0.05 ml patients (n = 16) | IVB 0.05 ml patients (n = 48) | p-value* | |
|---|---|---|---|
| △ Corneal Thickness (Pre-Post) | -28.1 ± 11.1 | -33.0 ± 9.2 | 0.163 |
| △ ACD (Pre-post) | -0.01 ± 0.05 | 0.10 ± 0.43 | 0.430 |
| △ ACV (Pre-post) | 5.8 ± 9.5 | 7.1 ± 9.6 | 0.855 |
| △ ACA (Pre-Post) | 2.4 ± 3.2 | 0.8 ± 4.0 | 0.288 |
| △ IOP (I) | 22.1 ± 8.6 | 20.7 ± 7.2 | 0.640 |
| △ IOP (5) | 4.8 ± 7.4 | 5.4 ± 6.3 | 0.807 |
Table 5.
Comparison of high IOP (≥21 mm Hg) patients at 5 minutes after intravitreal injection
| IVTA 0.05 ml patients (n = 4) | IVB 0.05 ml patients (n = 22) | p-value* | |
|---|---|---|---|
| △ Corneal thickness (Pre-post) | -29.0 ± 5.7 | -37.8 ± 11.1 | 0.309 |
| △ ACD (Pre-post) | -0.02 ± 0.02 | -0.03 ± 0.07 | 0.746 |
| △ ACV (Pre-post) | -1.5 ± 17.7 | 11.2 ± 23.5 | 0.489 |
| △ ACA (Pre-Post) | -1.0 ± 5.7 | 2.3 ± 3.6 | 0.279 |
| △ IOP (5) | 14.5 ± 10.6 | 11.5 ± 4.8 | 0.506 |
Table 6.
Comparison between high IOP (≥21 mm Hg) and normal IOP (<21 mm Hg) patients at 5 minutes after intravitreal injection
|
IVTA 0.05 ml patients (n = 16) |
IVB patients (n = 48) |
|||||
|---|---|---|---|---|---|---|
| Normal IOP (n = 12) | High IOP (n = 4) | p-value* | Normal IOP (n = 26) | High IOP (n = 22) | p-value* | |
| △ Corneal thickness (Pre-post) | -27.8 ± 12.8 | -29.0 ± 5.7 | 0.909 | -30.2 ± 6.0 | -37.8 ± 11.1 | 0.058 |
| △ ACD (Pre-post) | -0.002 ± 0.06 | -0.02 ± 0.02 | 0.790 | 0.19 ± 0.54 | -0.03 ± 0.07 | 0.238 |
| △ ACV (Pre-post) | 8.2 ± 6.0 | -1.5 ± 17.7 | 0.239 | 4.3 ± 17.1 | 11.2 ± 23.5 | 0.393 |
| △ ACA (Pre-Post) | 3.6 ± 1.2 | -1.0 ± 5.7 | 0.070 | 0.1 ± 3.8 | 2.3 ± 3.6 | 0.144 |
| △ IOP (5) | 1.5 ± 3.1 | 14.5 ± 10.6 | 0.001 | 1.2 ± 2.6 | 11.5 ± 4.8 | 0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download