Abstract
Purpose
To compare the anatomical and functional results of vitrectomy for macular hole with and without prone posture.
Methods
We retrospectively reviewed the medical records of 71 eyes of 71 patients who received macular hole repair and were followed up for at least 6 months. The anatomical success, complications, and best corrected visual acuity at post- operative 6 months and last follow-up between patients who were advised to take a prone posture for 1 week (group 1) and patients who were advised to simply avoid the supine position right from the surgery (group 2) were analyzed. Subgroup division analysis according to macular hole size and concurrent phacoemulsification was performed.
Results
Macular hole closure rate was 91.7% (33 of 36 eyes) in group 1 and 88.6% (31 of 35 eyes) in group 2 (p=0.710). The mean visual acuity at final follow-up increased in both groups by 4.75 ± 3.83 and 4.76 ± 2.96 lines, respectively and revealed no statistically significant difference (p = 0.988). Twenty-seven of 36 eyes (75%) in group 1 and 30 of 35 eyes (85.7%) in group 2 underwent concurrent phacoemulsification, and no difference in macular hole closure rate and visual acuity improvement between the two postures was observed.
Go to : 
References
1. la Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand. 2002; 80:579–87.

2. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991; 109:654–9.

3. Glaser BM, Michels RG, Kuppermann BD, et al. Transforming growth factor-beta 2 for the treatment of full-thickness macular holes. A prospective randomized study. Ophthalmology. 1992; 99:1162–72. discussion 1173.
4. Liggett PE, Skolik DS, Horio B, et al. Human autologous serum for the treatment of full-thickness macular holes. A preliminary study. Ophthalmology. 1995; 102:1071–6.
5. Gaudric A, Massin P, Paques M, et al. Autologous platelet concen- trate for the treatment of full-thickness macular holes. Graefes Arch Clin Exp Ophthalmol. 1995; 233:549–54.
6. Gaudric A, Haouchine B, Massin P, et al. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol. 1999; 117:744–51.
7. Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical co- herence tomography. Ophthalmology. 2001; 108:15–22.
8. Niwa H, Terasaki H, Ito Y, Miyake Y. Macular hole development in fellow eyes of patients with unilateral macular hole. Am J Ophthalmol. 2005; 140:370–5.

9. Uemoto R, Yamamoto S, Takeuchi S. Epimacular proliferative re- sponse following internal limiting membrane peeling for idio- pathic macular holes. Graefes Arch Clin Exp Ophthalmol. 2004; 242:177–80.
10. Hirneiss C, Neubauer A, Gass C, et al. Visual quality of life after macular hole surgery: outcome and predictive factors. Br J Ophthalmol. 2007; 91:481–4.

11. Scott IU, Moraczewski AL, Smiddy WE, et al. Long-term anatom- ic and visual acuity outcomes after initial anatomic success with macular hole surgery. Am J Ophthalmol. 2003; 135:633–40.
12. Smiddy WE, Pimentel S, Williams GA. Macular hole surgery with- out using adjunctive additives. Ophthalmic Surg Lasers. 1997; 28:713–7.
13. Holekamp NM, Meredith TA, Landers MB, et al. Ulnar neuropathy as a complication of macular hole surgery. Arch Ophthalmol. 1999; 117:1607–10.

14. Eckardt C, Eckert T, Eckardt U, et al. Macular hole surgery with air tamponade and optical coherence tomography-based duration of face-down positioning. Retina. 2008; 28:1087–96.

15. Guillaubey A, Malvitte L, Lafontaine PO, et al. Comparison of face-down and seated position after idiopathic macular hole sur- gery: a randomized clinical trial. Am J Ophthalmol. 2008; 146:128–34.
16. Gupta D. Face-down posturing after macular hole surgery: a review. Retina. 2009; 29:430–43.
17. Mittra RA, Kim JE, Han DP, Pollack JS. Sustained postoperative face-down positioning is unnecessary for successful macular hole surgery. Br J Ophthalmol. 2009; 93:664–6.

18. Solebo AL, Lange CA, Bunce C, Bainbridge JW. Face-down positioning or posturing after macular hole surgery. Cochrane Database Syst Rev. 2011; (12):CD008228.

19. Tadayoni R, Vicaut E, Devin F, et al. A randomized controlled trial of alleviated positioning after small macular hole surgery. Ophthalmology. 2011; 118:150–5.

20. Tatham A, Banerjee S. Face-down posturing after macular hole surgery: a meta-analysis. Br J Ophthalmol. 2010; 94:626–31.

21. Tornambe PE, Poliner LS, Grote K. Macular hole surgery without face-down positioning. A pilot study. Retina. 1997; 17:179–85.
22. Wickens JC, Shah GK. Outcomes of macular hole surgery and shortened face down positioning. Retina. 2006; 26:902–4.

23. Yagi F, Sato Y, Takagi S, Tomita G. Idiopathic macular hole vi- trectomy without postoperative face-down positioning. Jpn J Ophthalmol. 2009; 53:215–8.
24. Thompson JT, Smiddy WE, Glaser BM, et al. Intraocular tampo- nade duration and success of macular hole surgery. Retina. 1996; 16:373–82.
25. Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology. 2001; 108:1471–6. discussion 1477-8.

27. Jumper JM, Gallemore RP, McCuen BW 2nd, Toth CA. Features of macular hole closure in the early postoperative period using optical coherence tomography. Retina. 2000; 20:232–7.

28. Karia N, Laidlaw A, West J, et al. Macular hole surgery using sili- cone oil tamponade. Br J Ophthalmol. 2001; 85:1320–3.
29. Demols P, Schrooyen M. [Analysis of optical coherence tomog- raphy for macular hole closure after surgery]. Bull Soc Belge Ophtalmol. 2003; (288):25–9.
30. Sato H, Kawasaki R, Yamashita H. Observation of idiopathic full-thickness macular hole closure in early postoperative period as evaluated by optical coherence tomography. Am J Ophthalmol. 2003; 136:185–7.

31. Wu D, Ho L, Lai M, Capone A Jr. Surgical outcomes of idiopathic macular hole repair with limited postoperative positioning. Retina. 2011; 31:609–11.

32. Merkur AB, Tuli R. Macular hole repair with limited nonsupine positioning. Retina. 2007; 27:365–9.

33. Simcock PR, Scalia S. Phacovitrectomy without prone posture for full thickness macular holes. Br J Ophthalmol. 2001; 85:1316–9.

34. Dhawahir-Scala FE, Maino A, Saha K, et al. To posture or not to posture after macular hole surgery. Retina. 2008; 28:60–5.

35. Rubinstein A, Ang A, Patel CK. Vitrectomy without postoperative posturing for idiopathic macular holes. Clin Experiment Ophthalmol. 2007; 35:458–61.

36. Thompson JT, Glaser BM, Sjaarda RN, Murphy RP. Progression of nuclear sclerosis and long-term visual results of vitrectomy with transforming growth factor beta-2 for macular holes. Am J Ophthalmol. 1995; 119:48–54.

37. Ellis JD, Baines PS. Patient perspectives on macular hole surgery. Ophthalmology. 2002; 109:622–3.

38. Lee SB, Nam KY, Kim KN, Jo YJ. The surgical results of stage 2 and 3 macular hole with internal limiting membrane peeling and intravitreal air. J Korean Ophthalmol Soc. 2009; 50:1076–81.
39. Yooh HS, Brooks HL Jr, Capone A Jr, et al. Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am J Ophthalmol. 1996; 122:67–75.
Go to : 
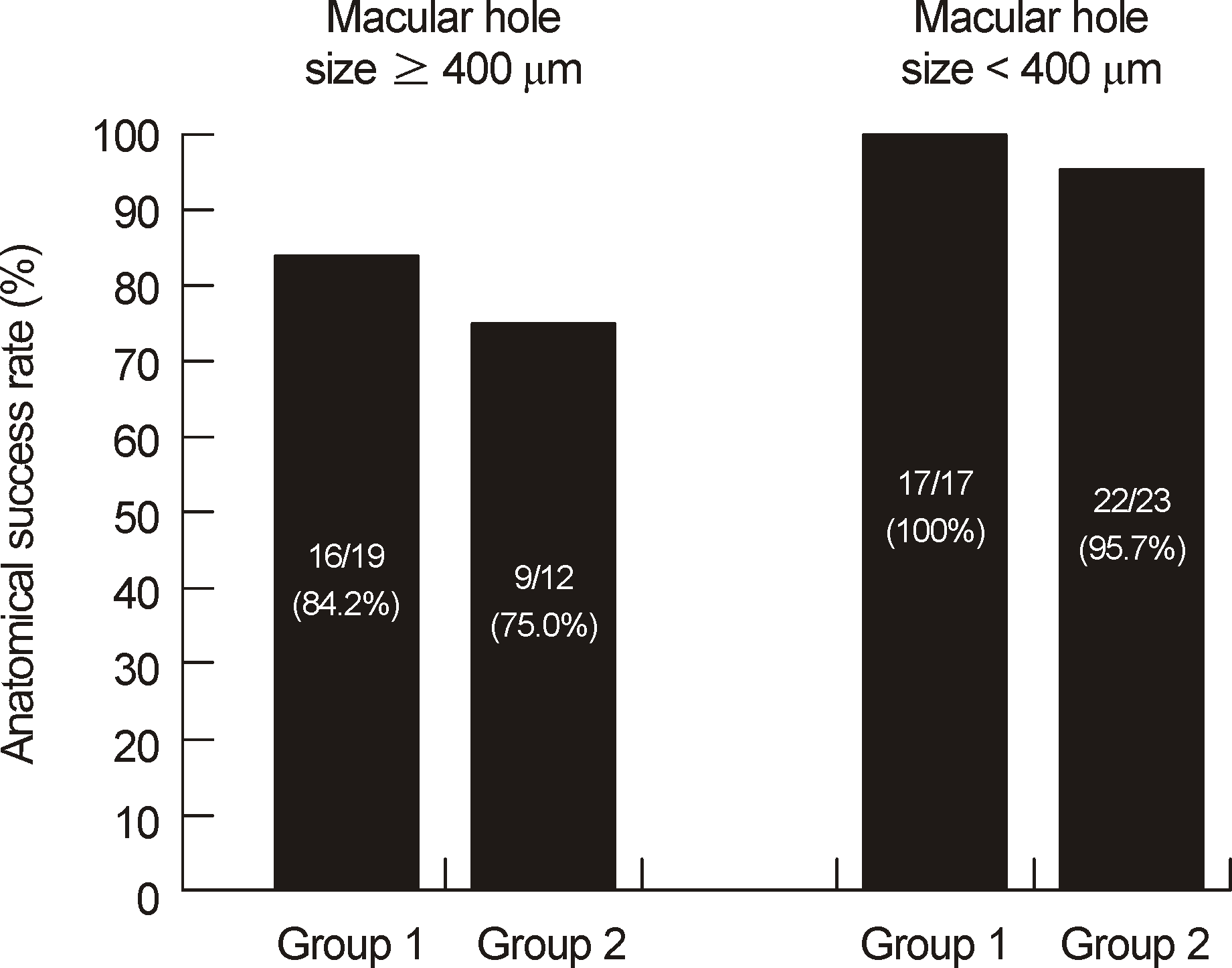 | Figure 1.Anatomical success rate between group with macular holes smaller than 400 μm and group with macular holes 400 μm or larger. There was no significant difference between prone and seated position in both groups (Fisher exact test; p = 0.653, p = 1.000). |
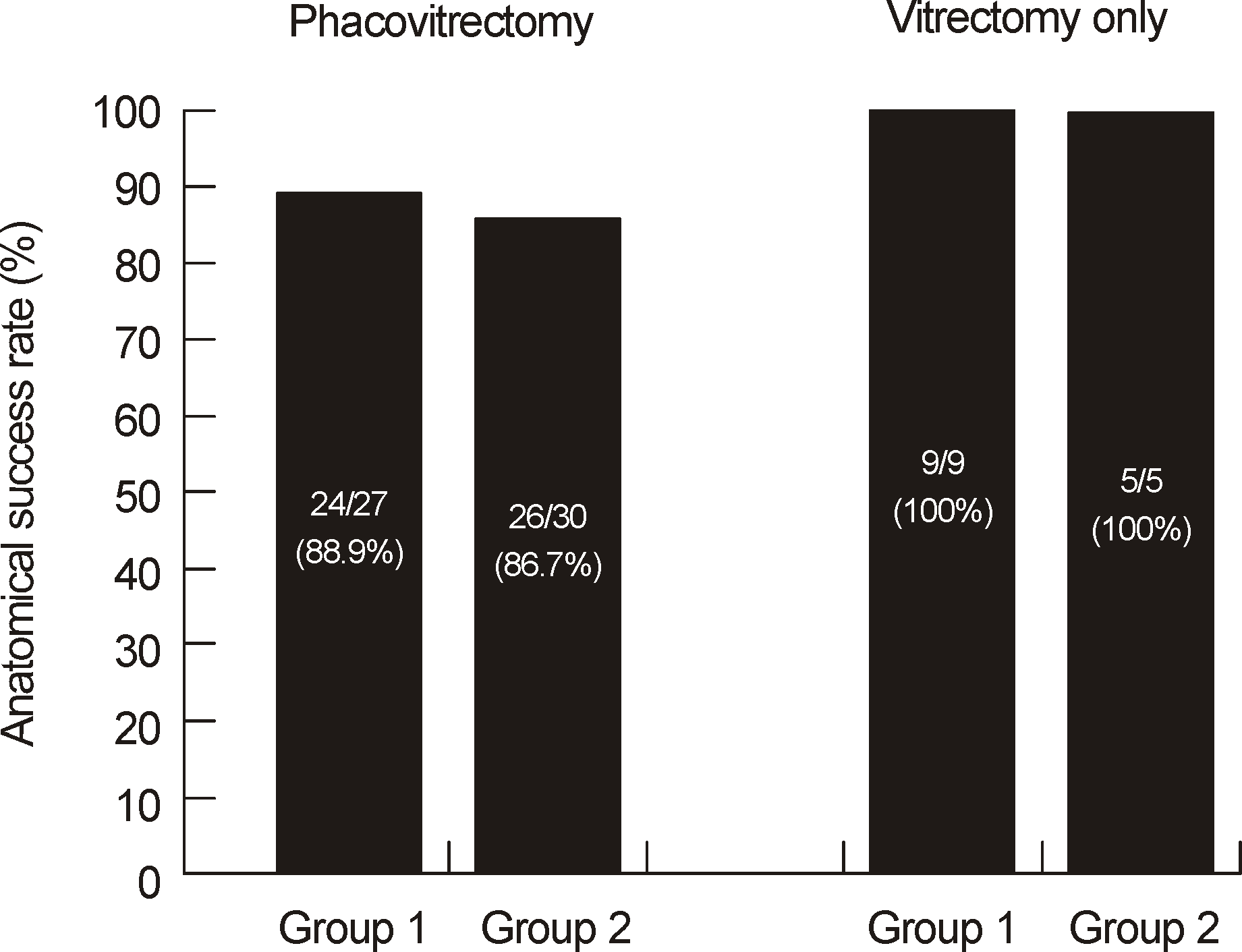 | Figure 2.Anatomical success rate between phacovitrectomy group and vitrectomy group. There was no significant difference between prone and seated position in both groups (Fisher's exact test & Pearson's Chi-square test; p = 1.00, p = 1.00). |
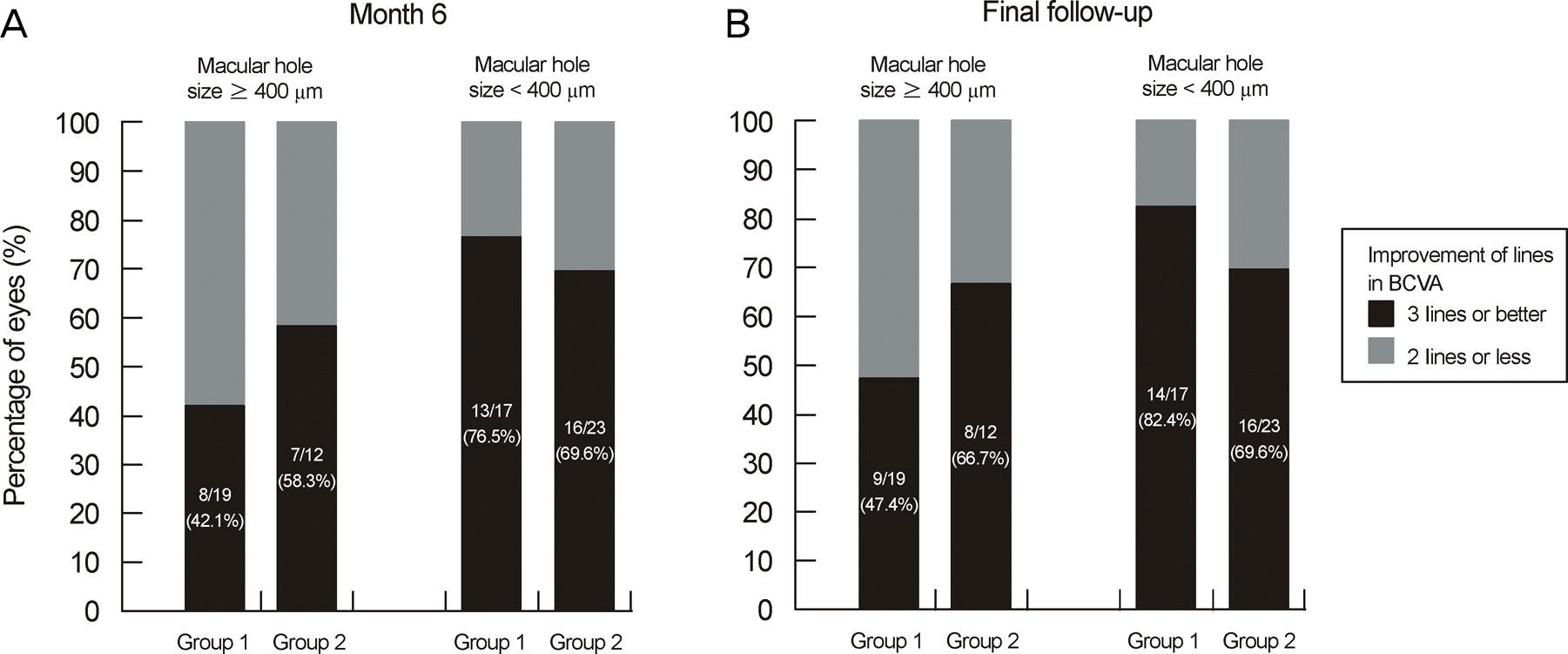 | Figure 3.Postoperative change in visual acuity at 6 month and final follow-up between group with macular holes smaller than 400 μm and group with macular holes 400 μm or larger. There was no significant difference between prone and seated position in each group (Fisher's exact test & Pearson's Chi-square test; p = 0.653, p = 0.629, p = 0.293, p = 0.471). |
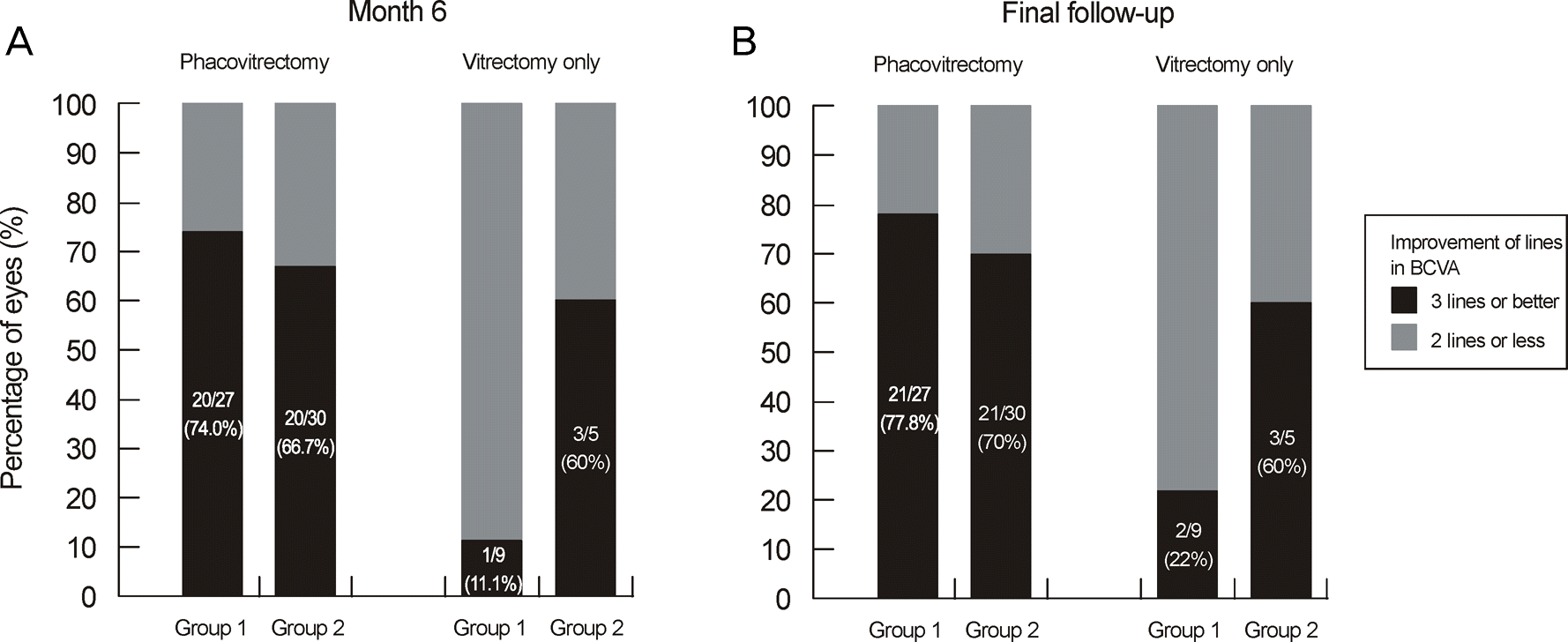 | Figure 4.Postoperative change in visual acuity between phacovitrectomy group and vitrectomy only group at 6 month and final follow-up. There was no significant difference between prone and seated position in each group at postoperative 6 months and final follow-up (Fisher's exact test & Pearson's Chi-square test; p = 0.542, p = 0.095, p = 0.506, p = 0.158). |
Table 1.
Patient demographics and trial data
Table 2.
Best corrected visual acuity and the number of eyes showed visual acuity improvement 3 lines or better
| Duration |
BCVA |
BCVA (3 lines or better) |
||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | p-value* | Group 1 | Group 2 | p-value† | |
| Preoperative | 0.99 ± 0.37 | 0.91 ± 0.35 | 0.309 | — | — | — |
| 6 months | 0.57 ± 0.28 | 0.47 ± 0.32 | 0.202 | 21 (58%) | 23 (66%) | 0.522 |
| Final | 0.52 ± 0.28 | 0.43 ± 0.34 | 0.230 | 23 (64%) | 24 (69%) | 0.677 |
Table 3.
Summary of postoperative complications
| Group 1 | Group 2 | p-value* | |
|---|---|---|---|
| Lens opacity increased, n† (%) | 6/9 (67) | 3/5 (60) | — |
| Posterior capsular opacity, n (%) | 7/27 (26) | 1/30 (3) | 0.478 |
| Posterior synechia, n (%) | 1/36 (3) | 0/35 (0) | 0.021 |
| Postoperative increased IOP, n (%) | 0/36 (0) | 4/35 (11) | 1.000 |
| Total, n (%) | 14/36 (39) | 8/35 (23) | 0.054 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download