Abstract
Purpose
To evaluate the 12-month outcomes of anti-vascular endothelial growth factor (VEGF) treatment for patients with retinal angiomatous proliferation (RAP).
Methods
Retrospective review of medical records was performed for 33 patients (33 eyes) who were diagnosed with RAP. All patients were initially treated with three consecutive intravitreal anti-VEGF injections after diagnosis. Additional treatment was performed when the recurrence of exudation was noted. The best-corrected visual acuity (BCVA) was measured before the first injection and at 3, 6, and 12 months after the first injection. The value measured before the treatment was compared with those measured after treatment.
Results
The patients received an average of 4.2 ± 1.7 intravitreal anti-VEGF injections during the 12-month follow-up period. The logarithm of minimal angle of resolution (log MAR) values of BCVA before the first injection and at 3, 6, and 12 months after the first injections were 0.76 ± 0.49, 0.55 ± 0.35, 0.67 ± 0.41, and 0.70 ± 0.50, respectively. BCVA was significantly improved at 3 and 6 months (p < 0.001 and p = 0.037) compared to that measured before the first injection. However, there was no significant difference between BCVA before the first injection and 12 months after the first injection (p = 0.590). At 12 months of follow-up, 29 eyes (87.9%) showed stable (<2 log MAR lines of change) or improved (52 log MAR lines of improvement) BCVA.
Go to : 
References
1. Yannuzzi LA, Negrâo S, Iida T, et al. Retinal angiomatous pro- liferation in age-related macular degeneration. Retina. 2001; 21:416–34.
2. Massacesi AL, Sacchi L, Bergamini F, Bottoni F. The prevalence of retinal angiomatous proliferation in age-related macular degener- ation with occult choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008; 246:89–92.
3. Maruko I, Iida T, Saito M, et al. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144:15–22.e2.

4. Liu Y, Wen F, Huang S, et al. Subtype lesions of neovascular age-related macular degeneration in Chinese patients. Graefe's Arch Clin Exp Ophthalmol. 2007; 245:1441–5.

5. Gross NE, Aizman A, Brucker A, et al. Nature and risk of neo- vascularization in the fellow eye of patients with unilateral retinal angiomatous proliferation. Retina. 2005; 25:713–8.
6. Bottoni F, Massacesi A, Cigada M, et al. Treatment of retinal an- giomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferation. Arch Ophthalmol. 2005; 123:1644–50.
7. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neo- vascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.
8. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus ver- teporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.
9. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treat- ment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.
10. Cohen SY, Dubois L, Tadayoni R, et al. Results of one-year's treat- ment with ranibizumab for exudative age-related macular degener- ation in a clinical setting. Am J Ophthalmol. 2009; 148:409–13.e1.
11. Kang S, Cho WK, Roh YJ. The efficacy of ranibizumab for choroi- dal neovascularization in age-related macular degeneration. J Korean Ophthalmol Soc. 2009; 50:725–30.
12. Kim YH, Kim ES, Yu SY, Kwak HW. Long-term effect of intra- vitreal bevacizumab for CNV secondary to age-related macular degeneration. J Korean Ophthalmol Soc. 2008; 49:1935–40.
13. Parodi MB, Iacono P, Menchini F, et al. Intravitreal bevacizumab versus ranibizumab for the treatment of retinal angiomatous proliferation. Acta Ophthalmol. 2013; 91:267–73.

14. Ghazi NG, Knape RM, Kirk TQ, et al. Intravitreal bevacizumab (Avastin) treatment of retinal angiomatous proliferation. Retina. 2008; 28:689–95.

15. Konstantinidis L, Mameletzi E, Mantel I, et al. Intravitreal ranibi- zumab (Lucentis®) in the treatment of retinal angiomatous pro- liferation (RAP). Graefes Arch Clin Exp Ophthalmol. 2009; 247:1165–71.
16. Costagliola C, Romano MR, dell'Omo R, et al. Intravitreal bev- acizumab for the treatment of retinal angiomatous proliferation. Am J Ophthalmol. 2007; 144:449–51.
17. Hufendiek K, Hufendiek K, Panagakis G, et al. Visual and morpho- logical outcomes of bevacizumab (Avastin®) versus ranibizumab (Lucentis®) treatment for retinal angiomatous proliferation. Int Ophthalmol. 2012; 32:259–68.
18. Kang JH, Park KA, Chung SE, Kang SW. Retinal angiomatous proliferation and intravitreal bevacizumab injection. Korean J Ophthalmol. 2007; 21:213–5.

19. Lee MY, Kim KS, Lee WK. Combination therapy of ranibizumab and photodynamic therapy for retinal angiomatous proliferation with serous pigment epithelial detachment in Korean patients: twelve-month results. Retina. 2011; 31:65–73.
20. Lee PY, Lee WK. Treatments of stage 1 retinal angiomatous proliferation. J Korean Ophthalmol Soc. 2008; 49:442–9.

21. Byeon SH, Hong JP, Lee H, et al. Photodynamic therapy results of retinal angiomatous proliferation with pigmented epithelial detach- ment in age-related macular degeneration. J Korean Ophthalmol Soc. 2006; 47:1410–6.
22. Cho YJ, Park SP. Intravitreal ranibizumab therapy for neovascular age-related macular degeneration with a predominantly hemor- rhagic lesion. J Korean Ophthalmol Soc. 2011; 52:838–45.
23. Chang MA, Do DV, Bressler SB, et al. Prospective one-year study of ranibizumab for predominantly hemorrhagic choroidal neo- vascular lesions in age-related macular degeneration. Retina. 2010; 30:1171–6.
24. Shienbaum G, Garcia Filho CA, Flynn HW Jr, et al. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapy. Am J Ophthalmol. 2013; 155:1009–13.

25. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in agerelated macular degeneration. Retina. 1996; 16:183–9.

26. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990; 109:33–7.

27. Riusala AM, Immonen IJ. Predictors of structural findings in old disciform lesions. Am J Ophthalmol. 2004; 138:245–53.

29. Arias L, Armada F, Donate J, et al. Delay in treating age-related macular degeneration in Spain is associated with progressive vision loss. Eye (Lond). 2009; 23:326–33.

30. Oliver-Fernandez A, Bakal J, Segal S, et al. Progression of visual loss and time between initial assessment and treatment of wet age-related macular degeneration. Can J Ophthalmol. 2005; 40:313–9.

31. Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina. 2012; 32:1260–4.
Go to : 
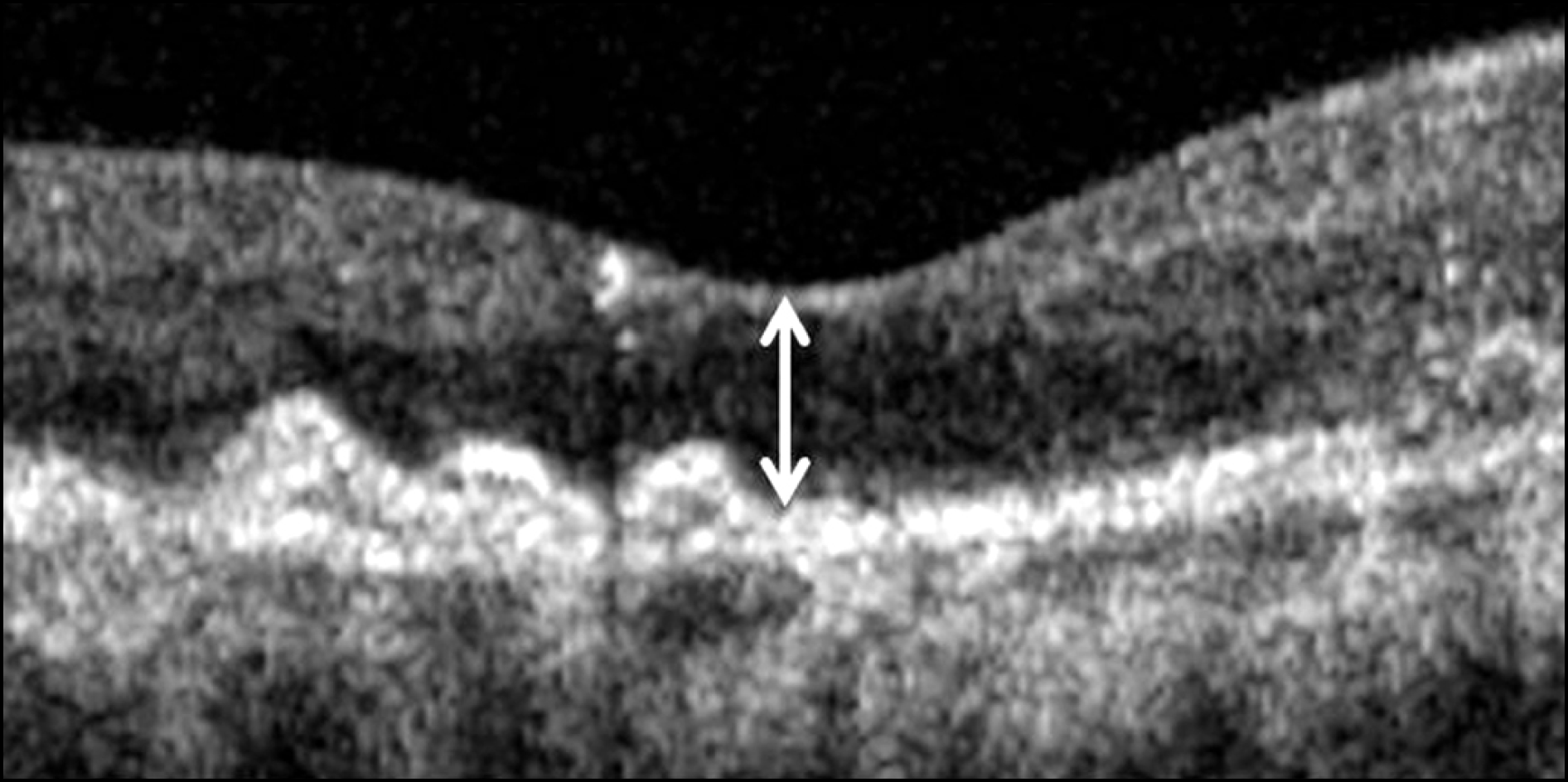 | Figure 1.Central foveal thickness (double-head arrow) was defined as the vertical distance between internal limiting membrane and retinal pigment epithelium at foveal center based on optical coherence tomography image centered at the center of the fovea. |
 | Figure 2.Fundus photography (A), indocyanine green angiography (B), and optical coherence tomography (C) images in an eye with retinal angiomatous proliferation. Arrow (B) on indocyanine green angiography image indicates retinal angiomatous proliferation lesion. Optical coherence tomography images showing macular microstructure before treatment (C1) and 3 months (C2), 8 months (C3), and 12 months (C4) after the first intravitreal anti-vascular endothelial growth factor injection. Recurrence of exudation was noted at 8 months following the first treatment. The best-corrected visual acuity measured before treatment was 20/40. The visual acuity was improved to 20/30 after three monthly injections, but deteriorated to 20/60 after the recurrence of exudation. At 12-month after the first treatment, the visual acuity was measured as 20/40. |
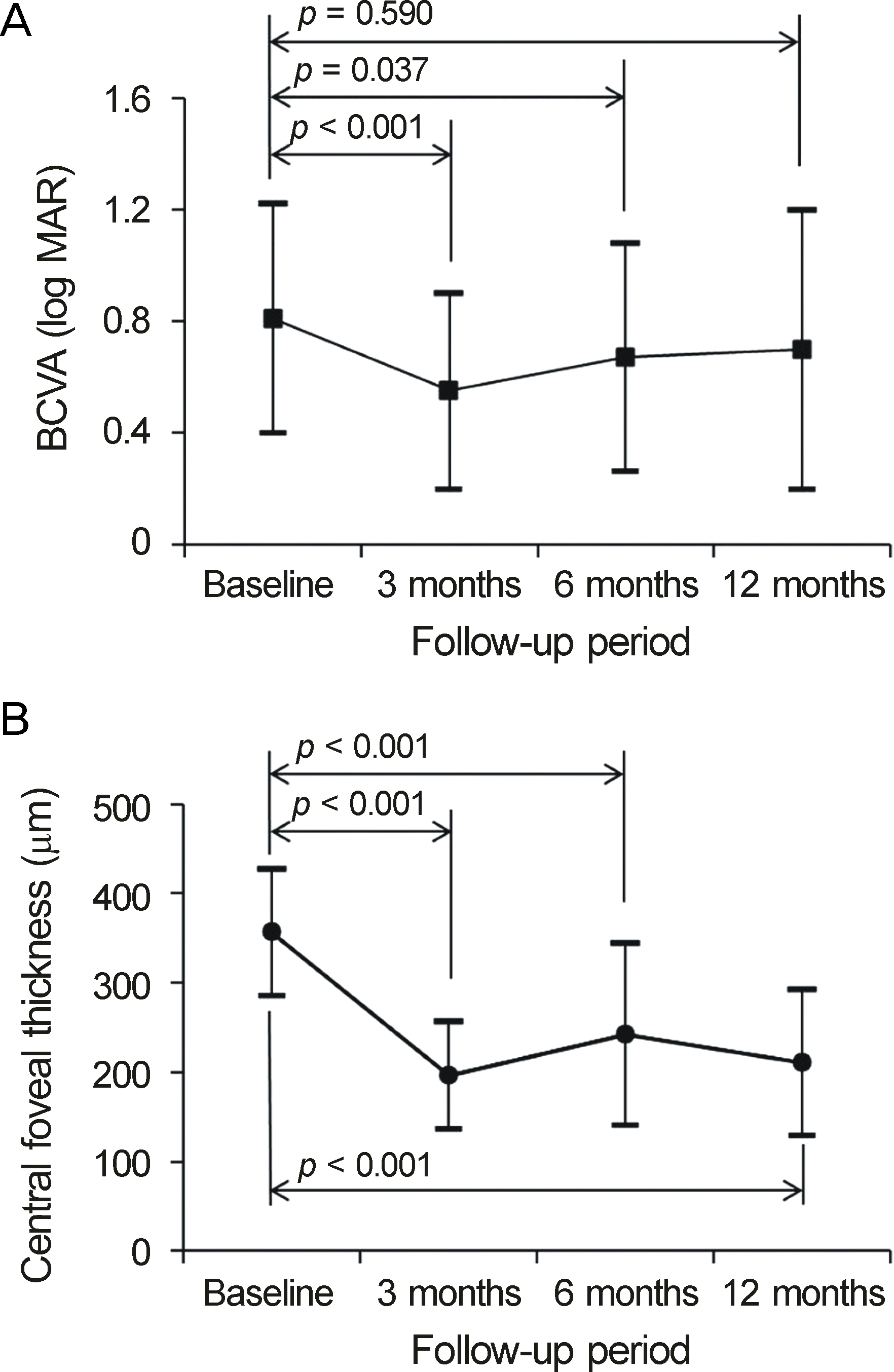 | Figure 3.Twelve-month changes in logarithm of minimal angle of resolution (log MAR) best-corrected visual acuity (A, BCVA) and central foveal thickness (B) in eyes with retinal angiomatous proliferation that were treated with anti-vascular growth factor mono-treatment, according to the follow-up period. Statistical analysis was performed using repeated measures analysis of variances. |
 | Figure 4.(A-D) Baseline fundus photography (A), fluorescein angiography (B), indocyanine green angiography (C), and optical coherence tomography (D) findings of an eye with retinal angiomatous proliferation. (E-G) Twelve months after anti-vascular endothelial growth factor treatment, resolution of retinal hemorrhage (E) cessation of leakage (F) and resolution of intraretinal and subretinal fluid (G) was noted. The visual acuity was improved from 20/400 to 20/100. |
Table 1.
Characteristics of 33 included eyes (33 patients) with retinal angiomatous proliferation
Table 2.
Distribution of eyes according to the degree of visual acuity change between at diagnosis and at 12-month following the first anti-vascular endothelial growth factor injection (N = 33)
| Degree of change | No. of eyes (%) |
|---|---|
| ≥0.2 log MAR of gain | 13 (39.4) |
| Stable | 16 (48.5) |
| ≥0.2 log MAR of loss | 4 (12.1) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download