Abstract
Purpose
To report a case of Vogt-Koyanagi-Harada (VKH) disease in a pregnant patient treated with intravitreal triamcinolone injection.
Case summary
A 21-year-old female in the 19th week of gestation presented with bilateral blurring of vision associated with mild headache and tinnitus. Her initial best corrected visual acuity was 0.15 in the right eye and 0.3 in the left eye. Multiple serous retinal detachment and anterior chamber inflammation were observed, and VKH disease was diagnosed. Because of her pregnancy, the patient did not want high-dose systemic prednisolone therapy which may cause an abortion or low birth weight infant when used in a pregnant patient. Therefore, an intravitreal triamcinolone (4 mg/0.1 ml) injection was given in the right eye and topical steroid eye drops were used in the left eye. After 1 day, serous retinal detachment was significantly decreased and anterior chamber inflammation disappeared in the right eye. After 1 week, no serous retinal detachment was observed. In the left eye, serous retinal detachment was decreased after using steroid eye drops. After 10 days, serous retinal detachment disappeared but anterior chamber inflammation was still observed. After 1 month, best corrected visual acuity was 1.0 in both eyes and serous retinal detachment had not recurred. On follow-up, VKH disease had not recurred and a healthy normal weight infant was delivered.
Go to : 
References
3. Reinisch JM, Simon NG, Karow WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978; 202:436–8.

4. Sawady J, Mercer BM, Wapner RJ. . The National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Beneficial Effects of Antenatal Repeated Steroids study: impact of repeated doses of antenatal corticosteroids on placental growth and histologic findings. Am J Obstet Gynecol. 2007; 197:281.e1–8.

5. Rao NA, Sukavatcharin S, Tsai JH. Vogt-Koyanagi-Harada disease diagnostic criteria. Int Ophthalmol. 2007; 27(2-3):195–9.

6. Nohara M, Norose K, Segawa K. Vogt-Koyanagi-Harada disease during pregnancy. Br J Ophthalmol. 1995; 79:94–5.

7. MacLean MA, Wilson R, Thomson JA. . Changes in immunologic parameters in normal pregnancy and spontaneous abortion. Am J Obstet Gynecol. 1991; 165(4 Pt 1):890–5.

8. Satoh A, Koh M, Tamura H. Harada's disease in pregnancy, a case report. Folia Ophthalmol Jpn. 1986; 37:460–5.
9. Sato S, Kou M, Tamaru H. Incomplete type Harada's disease in early pregnant stage treated with topical corticosteroid. Nippon Ganka Kiyu (Folia Ophthalmol Jpn). 1986; 37:46–50.
10. Taguchi C, Ikeda E, Hikita N, Mochizuki M. [A report oftwo cases suggesting positive influence of pregnancy on uveitis activity]. Nihon Ganka Gakkai Zasshi. 1999; 103:66–71.
11. Miyata N, Sugita M, Nakamura S. . Treatment of Vogt- Koyanagi-Harada's disease during pregnancy. Jpn J Ophthalmol. 2001; 45:177–80.
12. Reed L HM, Shlomo M, Keneth Sp. Williams text book of endocrinology. 10th ed.Philadelphia: Saunders;2003.
13. Friedman Z, Granat M, Neumann E. The syndrome of Vogt- Koyanagi-Harada and pregnancy. Metab Pediatr Ophthalmol. 1980; 4:147–9.
14. Wittig EO, Moreira CA, Basedow H. [Vogt-Koyanagi syndrome. Report of a case in a pregnant woman]. Arq Neuropsiquiatr. 1983; 41:385–90.
15. Steahly LP. Vogt-Koyanagi-Harada syndrome and pregnancy. Ann Ophthalmol. 1990; 22:59–62.
16. Dombrowski MP, Brown CL, Berry SM. Preliminary experience with triamcinolone acetonide during pregnancy. J Matern Fetal Med. 1996; 5:310–3.

17. Degenring RF, Jonas JB. Serum levels of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004; 137:1142–3.

18. Byon IS, Kim JH, Lee JE, Oum BS. Intravitreal triamcinolone ace- tonide for rebound phenomenon after high-dose intravenous steroid treatment in Vogt-Koyanagi-Harada disease. Clin Ophthalmol. 2011; 5:1589–91.
Go to : 
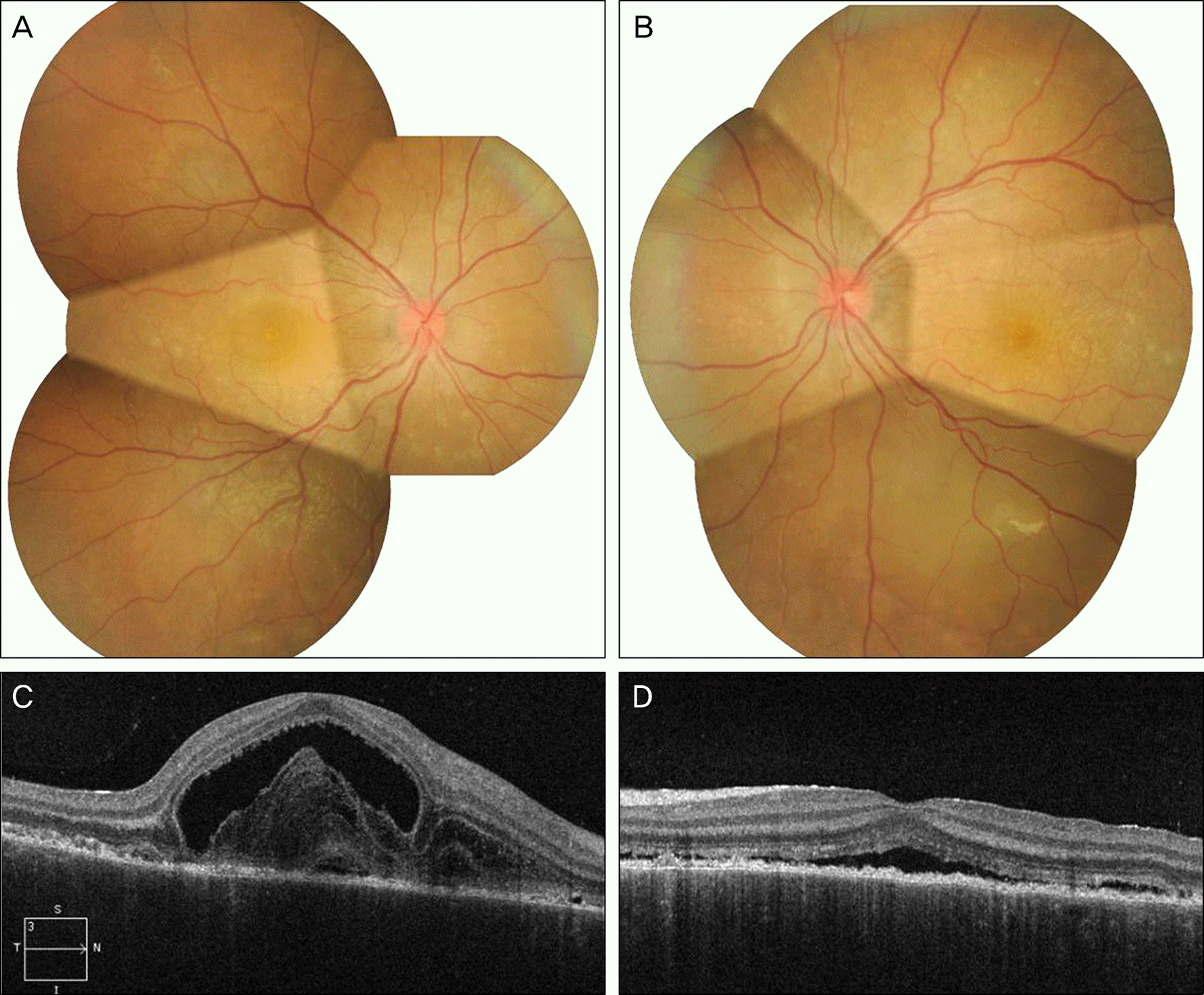 | Figure 1.Fundus photographs and optical coherence tomography of patient at presentation (A, C: right eye; B, D: left eye). |
 | Figure 2.Serial optical coherence tomography (OCT) during follow-up period. (A) Before intravitreal triamcinolone injection and using corticosteroid eye drops. (B) One day after intravitreal triamcinolone injection to right eye. OCT shows significant reduction of serous retinal detachment. (C) Three days after treatment. In the left eye, serous retinal detachment started to decrease. (D) Ten days after treatment, relatively flat retina observed in both eyes. (E) One month after treatment. Complete resolution of serous retinal detachment. But in the left eye anterior chamber inflammationis still observed. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download