Abstract
Purpose
Intravitreal anti-vascular endothelial growth factor (VEGF) treatment has become an important part in the treatment of neovascular age-related macular degeneration (AMD). In the present study we describe the clinical feature of retinal pigment epithelium (RPE) tears after intravitreal injection of anti-VEGF agent and compared the tear group to the control group.
Methods
In this retrospective case series, data of 11 patients with eyes that developed RPE tears after intravitreal anti-VEGF injection (8 ranibizumab and 3 bevacizumab) were collected and analyzed. The tear group included 11 patients with eyes that developed RPE tears and the control group included 22 patients with no RPE tears after treatment. We investigated age, gender, bilaterality, duration from injection to tear, pigment epithelial detachment (PED) height and diameter, along with central retinal thickness (CMT) using optical coherence tomography (OCT), fluorescein angiography (FAG), and visual acuities before and after treatment.
Results
The mean age of the tear group was 81.36 ± 5.55 years which was significantly different from the control group's mean age of 74.82 ± 5.28 years (p = 0.003). OCT findings showed PED greatest linear dimension (GLD) was 2978.45 ± 947.69 μm in the tear group and 2250.23 ± 988.49 μm in the control group (p = 0.027). PED height was 507.09 ± 153.97 μm in the tear group and 353.23 ± 199.42 μm in the control group (p = 0.010). CMT was 431.64 ± 200.33 μm in the tear group and 289.95 ± 61.27 μm in the control group (p = 0.005). There was no significant difference between groups according to gender, bilaterality, visual acuities, and subretinal fluid based on OCT and FAG findings. In the tear group, visual acuities before and after the tear were not significantly different.
Go to : 
References
1. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Stydy. Ophthalmology. 1992; 99:933–43.
2. Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102:1640–2.

3. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005; 25:111–8.

4. Ferrara N, Damico L, Shams N. . Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–70.

5. Song MH, Kim JY, Roh YJ. Short-term efficacy of intravitreal ranibizumab for myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2009; 50:1027–34.

6. Bashshur ZF, Haddad ZA, Schakal A. . Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol. 2008; 145:249–56.

7. Mozayan A, Farah S. Acute anterior uveitis following intravitreal injection of bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:25–7.

8. Skalicky SE, Ho I, Agar A, Bank A. Glaucoma filtration surgery following sustained elevation of intraocular pressure secondary to intravitreal anti-VEGF injections. Ophthalmic Surg Lasers Imaging. 2012; 43:328–34.

9. Jamrozy-Witkowska A, Kowalska K, Jankowska-Lech I. . [Complications of intravitreal injections–own experience]. Klin Oczna. 2011; 113(4-6):127–31.
10. Hoskin A, Bird AC, Sehmi K. Tears of detached retinal pigment epithelium. Br J Ophthalmol. 1981; 65:417–22.

11. Meyer CH, Toth CA. Retinal pigment epithelial tear with vitre- omacular attachment: a novel pathogenic feature. Graefes Arch Clin Exp Ophthalmol. 2001; 239:325–33.
12. Gamulescu MA, Framme C, Sachs H. RPE-rip after intravitreal bevacizumab (Avastin) treatment for vascularised PED secondary to AMD. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1037–40.

13. Hartnett ME, Lappas A, Darland D. . Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003; 77:593–9.

14. Krishan NR, Chandra SR, Stevens TS. Diagnosis and pathogenesis of retinal pigment epithelial tears. Am J Ophthalmol. 1985; 100:698–707.

15. Cantrill HL, Ramsay RC, Knobloch WH. Rips in the pigment epithelium. Arch Ophthalmol. 1983; 101:1074–9.

16. Gass JD. Retinal pigment epithelial rip during krypton red laser photocoagulation. Am J Ophthalmol. 1984; 98:700–6.
17. Yeo JH, Marcus S, Murphy RP. Retinal pigment epithelial tears. Patterns and prognosis. Ophthalmology. 1988; 95:8–13.
18. Decker WL, Sanborn GE, Ridley M. . Retinal pigment epithelial tears. Ophthalmology. 1983; 90:507–12.

19. Gass JD. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol. 1984; 68:513–9.

20. Gass JD. Serous retinal pigment epithelial detachment with a notch. A sign of occult choroidal neovascularization. Retina. 1984; 4:205–20.
21. Chang LK, Sarraf D. Tears of the retinal pigment epithelium: an old problem in a new era. Retina. 2007; 27:523–34.
22. Gass JD. Idiopathic central serous chorioretinopathy. Stereoscopic atlas of macular diseases: diagnosis and treatment. St. Louis: Mosby;1997. p. 52–70.
23. Schoeppner G, Chuang EL, Bird AC. The risk of fellow eye visual loss with unilateral retinal pigment epithelial tears. Am J Ophthalmol. 1989; 108:683–5.

24. Chuang EL, Bird AC. Bilaterality of tears of the retinal pigment epithelium. Br J Ophthalmol. 1988; 72:918–20.

25. Chan CK, Meyer CH, Gross JG. . Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007; 27:541–51.

26. Cunningham ET Jr, Feiner L, Chung C. . Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. Ophthalmology. 2011; 118:2447–52.

27. Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985; 69:397–403.

28. Pauleikhoff D, Löffert D, Spital G. . Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol. 2002; 240:533–8.
Go to : 
 | Figure 1.A vision preserved case. The foveal area is saved from the missing RPE bed area after the RPE tear had occurred (right). The contracted sheath of RPE is under the foveal area. Visual acuity is increased. |
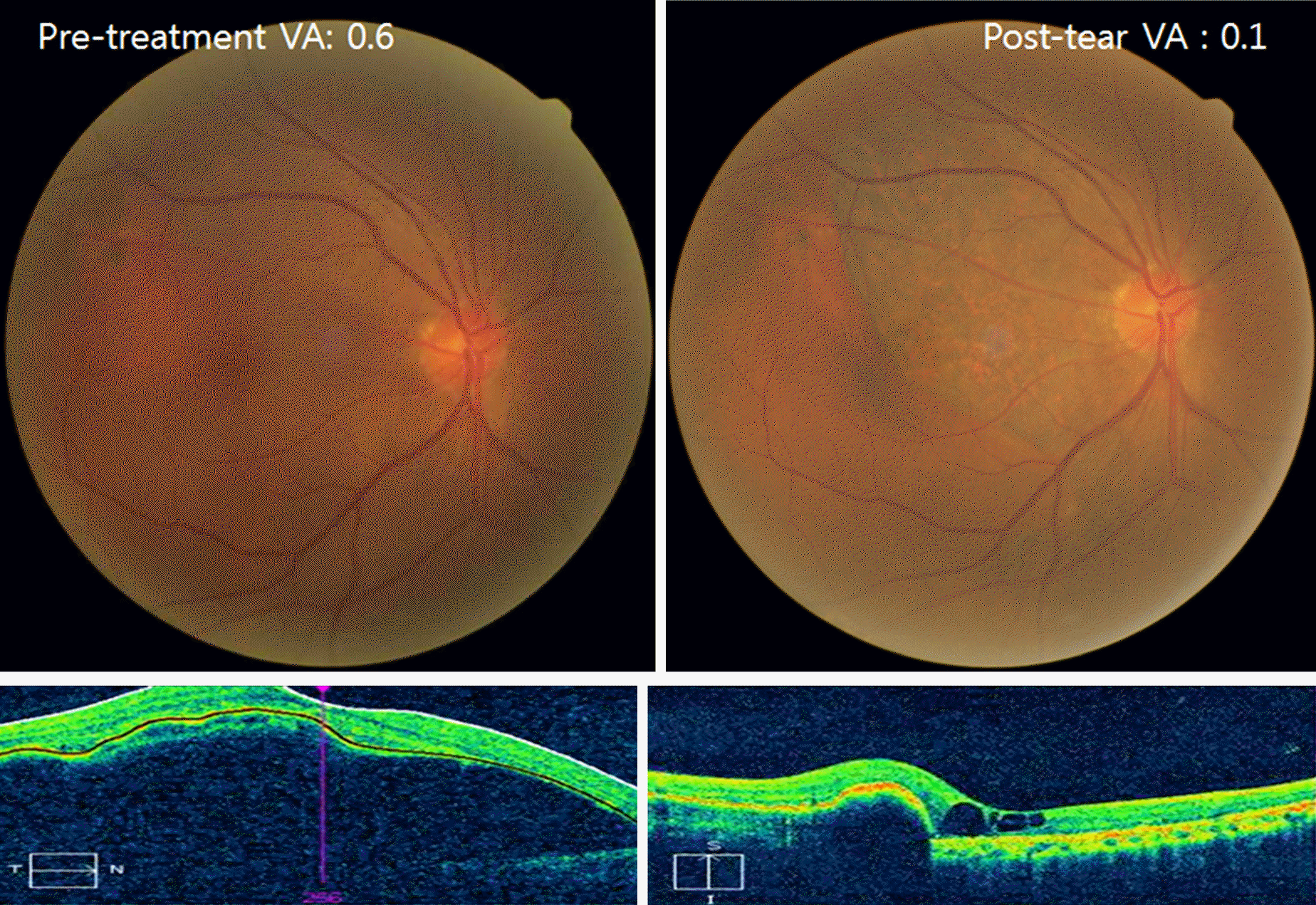 | Figure 2.A vision deprived case. Fundus photo and OCT demonstrates a large PED before treatment (left) and RPE tear after treatment (right). In the right image, the fovea is on the RPE missing area and has cysts on OCT image. Visual acuity is seriously decreased. |
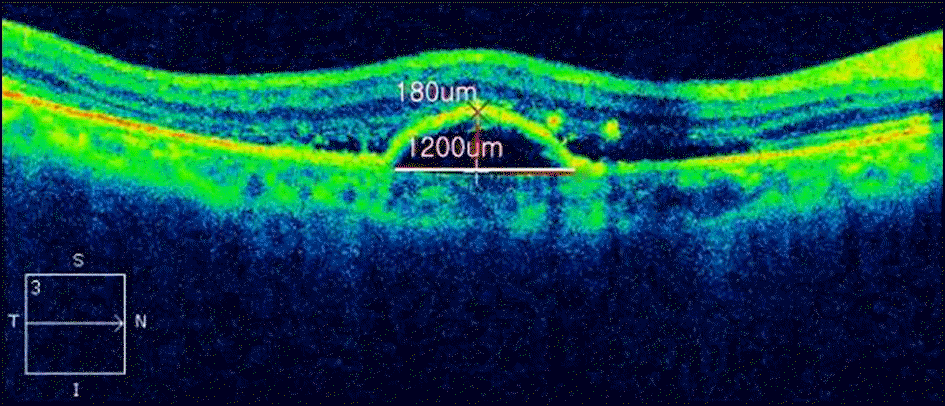 | Figure 3.OCT image measuring of PED height and diameter by using of caliper function in SD-OCT (CIRRUS™ HD-OCT 4000, Carl Zeiss Meditec, Dublin, CA, USA). |
Table 1.
Charicteristics of 11 patients with RPE tear after treatments
Table 2.
Comparison of characteristics between tear group and control group treated with anti-VEGF intravitreal injection
| Tear group | Control group | p-value* | |
|---|---|---|---|
| Age (years) | 81.36 ± 5.55 | 74.82 ± 5.28 | 0.003 |
| Sex (M/F) | 5/6 | 12/10 | 0.628 |
| OD / OS | 7/4 | 13/9 | 0.804 |
Table 3.
Comparison of visual acuities between groups before and after the treatment
| Tear group | Control group | p-value† | |
|---|---|---|---|
| Pre-treatment | 1.26 ± 0.74* | 1.12 ± 0.79* | 0.687 |
| Post-treatment | 1.43 ± 0.74* | 0.91 ± 0.69* | 0.058 |
Table 4.
In the tear group, changes of visual acuity before and after the tear
| VA (log MAR) | |
|---|---|
| Pre-tear | 1.36 ± 0.78* |
| Post-tear | 1.43 ± 0.74* |
| p-value† | 0.160 |
Table 5.
Comparisons between tear group and control group on OCT findings
| Tear group | Control group | p-value* | |
|---|---|---|---|
| PED diameter (μm) | 2978.45 ± 947.69 | 2250.23 ± 988.49 | 0.027 |
| PED height (μm) | 507.09 ± 153.97 | 353.23 ± 199.42 | 0.010 |
| CMT (μm) | 431.64 ± 200.33 | 289.95 ± 61.27 | 0.005 |
| SRF (+/-) | 7/4 | 16/6 | 0.598 |
Table 6.
Comparisons between tear group and control group about PED and CNV size on FAG image
| Tear group | Control group | p-value* | |
|---|---|---|---|
| PED area (mm2) | 19.87 ± 18.35 | 8.87 ± 18.35 | 0.061 |
| PED GLD (mm) | 4.88 ± 2.70 | 3.41 ± 1.41 | 0.093 |
| CNV area (mm2) | 2.75 ± 3.50 | 1.12 ± 1.80 | 0.061 |
| CNV GLD (mm) | 1.74 ± 1.28 | 1.17 ± 0.68 | 0.086 |
| CNV/PED (area) | 0.19 ± 0.20 | 0.18 ± 0.21 | 0.730 |
| CNV/PED (GLD) | 0.46 ± 0.30 | 0.38 ± 0.18 | 0.579 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download