Abstract
Purpose
To determine the risk factors of retinal pigment epithelial (RPE) tears developed after treatment of exudative age-related macular degeneration (AMD) and to report its clinical manifestations.
Methods
A retrospective, consecutive chart review was performed for all patients with exudative AMD treated with intra- vitreal anti-vascular endothelial growth factor (VEGF) antibody or photodynamic therapy (PDT) between March 2010 and January 2013. The main outcome measures were the time from first injection to development of the RPE tear and pre- and post-RPE tear visual acuity. The visual acuity conservational interval was defined between the time the RPE tear occurred and the time visual acuity decreased 5 letters or more from pre-RPE tear visual acuity.
Results
A total of 219 eyes were treated with intravitreal bevacizumab, ranibizumab or PDT. Ten eyes from 10 patients developed a RPE tear (4.6%); 7 were occult choroidal neovasculization (CNV) and 3 were polypoidal choroidal vasculop- athy (PCV). The average age of the RPE tear patients was 75.4 years which is statistically greater than the others (65.7) (p = 0.001). Ninety percent (9/10) of the RPE tears occurred within the first 12 weeks after treatment started. Five patients conserved their visual acuity for approximately 4 months after the RPE tear occurred. However, all 10 patients had poor visual acuity within 1 year of the follow-up period.
Go to : 
References
1. Gass JD. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol. 1984; 68:513–9.

2. Hoskin A, Bird AC, Sehmi K. Tears of detached retinal pigment epithelium. Br J Ophthalmol. 1981; 65:417–22.

3. Caswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985; 69:397–403.
4. Gass JD. Retinal pigment epithelial rip during krypton red laser photocoagulation. Am J Ophthalmol. 1984; 98:700–6.
5. Pece A, Isola V, Vadalà M, Calori G. Photodynamic therapy with verteporfin for choroidal neovascularization associated with retinal pigment epithelial detachment in age-related macular degeneration. Retina. 2007; 27:342–8.

6. Pece A, Introini U, Bottoni F, Brancato R. Acute retinal pigment epithelial tear after photodynamic therapy. Retina. 2001; 21:661–5.

7. Axer-Siegel R, Ehrlich R, Rosenblatt I. . Photodynamic therapy for occult choroidal neovascularization with pigment epithelium detachment in age-related macular degeneration. Arch Ophthalmol. 2004; 122:453–9.
8. Copt RP, Zografos L. Retinal pigment epithelial tear after photodynamic therapy for choroidal neovascularization caused by age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001; 42:S440.
9. Lommatzsch A, Radermacher M, Spital G, Pauleikhoff D. Photodynamic therapy of pigment epithelium detachments in AMD. Invest Ophthalmol Vis Sci. 2001; 42:S439.
10. Dhalla MS, Blinder KJ, Tewari A. . Retinal pigment epithelial tear following intravitreal pegaptanib sodium. Am J Ophthalmol. 2006; 141:752–4.

11. Garg S, Brod R, Kim D. . Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration. Clin Experiment Ophthalmol. 2008; 36:252–6.

12. Ronan SM, Yoganathan P, Chien FY. . Retinal pigment epithelium tears after intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Retina. 2007; 27:535–40.

13. Chan CK, Meyer CH, Gross JG. . Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007; 27:541–51.

14. Spandau UH, Jonas JB. Retinal pigment epithelium tear after intravitreal bevacizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2006; 142:1068–70.

15. Gelisken F, Ziemssen F, Voelker M, Bartz-Schmidt KU. Retinal pigment epithelial tear following intravitreal bevacizumab injection for neovascular age-related macular degeneration. Acta Ophthalmol Scand. 2006; 84:833–4.

16. Shah CP, Hsu J, Garg SJ. . Retinal pigment epithelial tear after intravitreal bevacizumab injection. Am J Ophthalmol. 2006; 142:1070–2.

17. Bashshur ZF, Bazarbachi A, Schakal A. . Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006; 142:1–9.

18. Avery RL, Pieramici DJ, Rabena MD. . Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.e5.

19. Apte RS. Retinal pigment epithelial tear after intravitreal ranibizumab for subfoveal CNV secondary to AMD. Int Ophthalmol. 2007; 27:59–61.

20. Chang LK, Sarraf D. Tears of the retinal pigment epithelium: an old problem a new era. Retina. 2007; 27:523–34.
21. Gutfleisch M, Heimes B, Schumacher M. . Long-term visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigment epithelial detachment in AMD. Eye (Lond). 2011; 25:1181–6.

22. Carvounis PE, Kopel AC, Benz MS. Retinal pigment epithelium tears following ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2007; 143:504–5.

23. Bakri SJ, Kitzmann AS. Retinal pigment epithelial tear after intravitreal ranibizumab. Am J Ophthalmol. 2007; 143:505–7.

24. Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neo vascularization-VIP report 2. Am J Ophthalmol. 2001; 131:541–60.
Go to : 
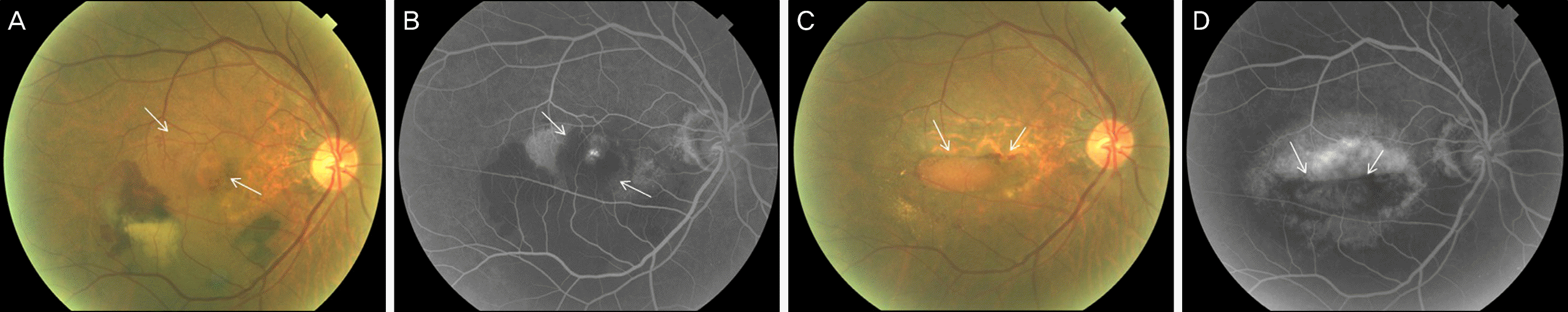 | Figure 1.This figure shows fundus photo & fluorescein angiography (FA) of patient 9. (A, B) There is a subfoveal choroidal neovascular membrane (arrows) before development of retinal pigment epithelial (RPE) tear. (C, D) RPE tear was developed and rolled RPE is shown (arrows). |
Table 1.
Summary of lesion type and visual outcomes before and after treatments
| Patient | Sex | Age | Lesion type | Treatment | Pre-Rxvision | 4-weeks F/U | 6-weeks F/U | 8-weeks F/U | 12-weeks F/U | 18-weeks F/U | 24-weeks F/U | 30-weeks F/U | 36-weeks F/U | 42-weeks F/U | 52-weeks F/U | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 70 | Occult,PED | Ranibizumab | 0.05 | 0.1 | 0.2 | Tear*,Ax3† | 0.2 | 0.2 | 0.2 | 0.05 | 0.05 | 0.05 | ||||
| 2 | M | 76 | Occult,PED | Ranibizumab | 0.15 | Tear,Lx2‡, Axl | 0.2 | 0.1 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | |||||
| 3 | M | 76 | Occult | Ranibizumab | 0.05 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | Tear,Axl | 0.03 | 0.03 | 0.03 | ||||
| 4 | F | 78 | Occult,PED | Ranibizumab | 0.2 | Tear, Lx2 | 0.1 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | |||||
| 5 | F | 73 | Occult,PED | Ranibizumab | 0.1 | Tear,Lx2, Ax7 | 0.2 | 0.2 | 0.15 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.04 | |||
| 6 | M | 76 | PCV, PED | Ranibizumab | 0.05 | Tear | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | |||||
| 7 | M | 81 | PCV,PED | Ranibizumab | 0.3 | 0.3 | 0.3 | Tear,Axl,Lx9 | 0.3 | 0.2 | 0.15 | 0.07 | 0.06 | 0.03 | 0.01 | |||
| 8 | F | 77 | Occult,PED | Bevacizumab | 0.2 | Tear, Ax5 | 0.2 | 0.06 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | ||||
| 9 | M | 72 | Occult,PED | PDT | 0.15 | 0.15 | 0.05 | Tear | 0.05 | 0.05 | 0.04 | 0.04 | 0.03 | 0.02 | ||||
| 10 | M | 75 | PCV,PED | Bevacizumab,PDT | 0.15 | 0.15 | Tear | 0.05 | 0.03 | 0.01 | 0.01 | 0.01 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download