Abstract
Purpose
To investigate the development of new choroidal neovascularization in fellow eyes of patients treated for unilateral exudative age-related macular degeneration (AMD).
Methods
Three hundred fourteen patients who were first diagnosed with unilateral exudative AMD and treated with intravitreal bevacizumab or ranibizumab were studied retrospectively.
Results
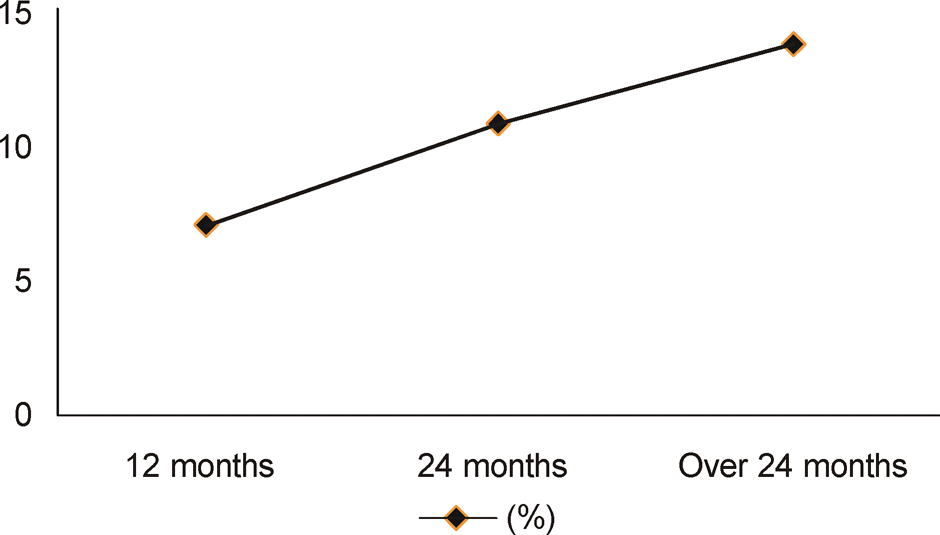
New exudative AMD developed in 7.0% of fellow eyes after 1 year, 10.8% after 2 years and 13.7% after more than 2 years. According to the subtype of exudative AMD, there were no significant differences between classic CNV, occult or minimally classic CNV, and PCV in the incidence of new exudative AMD. After 2 years, a higher conversion rate was found in the bevacizumab-treated group than the ranibizumab-treated group.
References
1. Friedman DS, O'Colmain BJ, Muñoz B. . Prevalence of age- related macular degeneration in the united states. Arch Ophthalmol. 2004; 122:556–72.
2. Kawasaki R, Yasuda M, Song JS. . The prevalance of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010; 117:921–7.
3. Park KH, Song SJ, Lee WK. . The results ofnation-wide registry of age-related macular degeneration in Korea. J Korean Ophthalmol Soc. 2010; 51:516–23.
4. Song SJ, Youm DJ, Chang Y, Yu HG. Age-related macular degeneration in a screened Korean population: prevalence, risk factors and subtypes. Ophthalmic Epidemiol. 2009; 16:304–10.
5. Klein R, Klein BE, Jensen SC, Meuer SM. The Five-year incidence and progression of age-related maculopahty: the Beaver Dam Eye Study. Ophthalmology. 1997; 104:7–21.
6. Klein R, Klein BE, Knudtson MD. . Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007; 114:253–62.
7. Mukesh BN, Dimitrov PN, Leikin S. . Five-year incidence of age-related maculopathy: the Visual Impariment Proj ect. Ophthalmology. 2004; 111:1176–82.
8. van Leeuwen R, Klaver CC, Vingerling JR. . The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003; 121:519–26.
9. Barbazetto IA, Saroj N, Shapiro H. . Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. Am J Ophthalmol. 2010; 149:939–46.e1.

10. Ueta T, Iriyama A, Francis J. . Development of typical age-related macular degeneration and polypoidal choroidal vasculopathy in fellow eyes of Japanese patients with exudative age-related macular degeneration. Am J Ophthalmol. 2008; 146:96–101.

11. Klaver CC, Assink JJ, van Leeuwen R. . Incidence and progression rate of age-related maculopathy: the Rotterdam study. Invest Ophthalmol Vis Sci. 2001; 42:2237–41.
12. Neri P, Mariotti C, Mercanti L, Salvolini S. . Vitritis in the contralateral uninjected eye following intravitreal bevacizumab (Avastin). Int Ophthalmol. 2008; 28:425–7.

13. Avery RL, Pearlman J, Pieramici DJ. . Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006; 113:1695.e1–15.

14. Sawada O, Kawamura H, Kakinoki M, Ohji M. Vascular endothelial growth factor in fellow eyes of eyes injected with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1379–81.

Figure 2.
Fundus photograph and fluorescein angiograph of the right eye of a 64 year old female (A, B, C); left eye (D, E, F). (A, B, C) No specific abnormality (D) Choroidal neovascular membrane (CNVM) of the macula with retinal hemorrhage. (E) Predominant classic choroidal neovascularizaion with well demarcated lesion 23 seconds after injection. (F) Late phase shows staining implying old lesions. She was treated with intravitreal Ranibizumab injection 3 times.
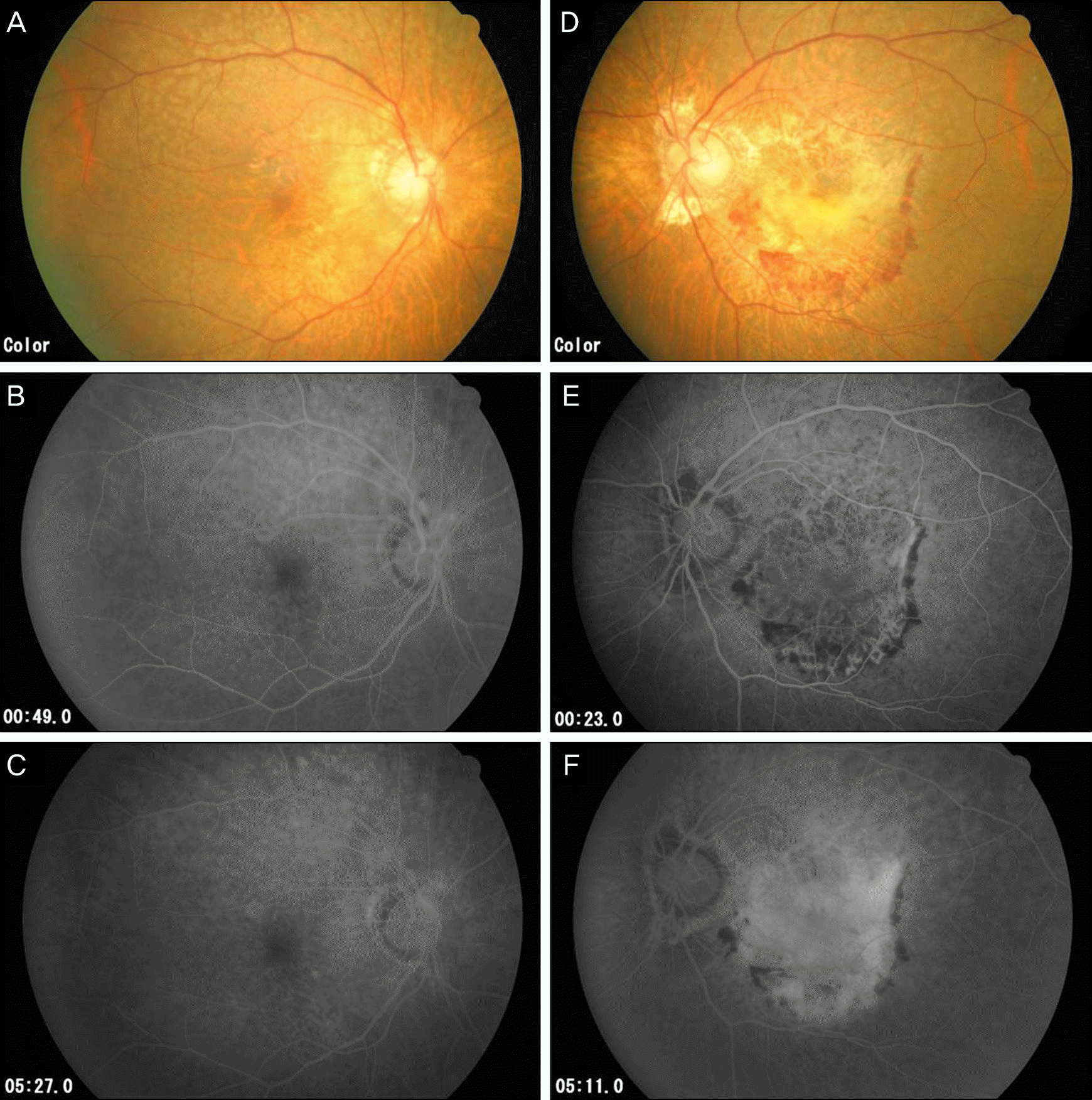
Figure 3.
Fundus photograph and fluorescein angiograph of same patient with figure 2 after 2 months of right visual disturbance. Right eye (A, B, C) and left eye (D, E, F). (A) New hemorrhagic spot around macula (B, C) Class choroidal neovascularization which was absent 2 months prior (D) Retinal hemorrhage was decreased with fibrotic tissue changes. (E, F) Scar change with staining.
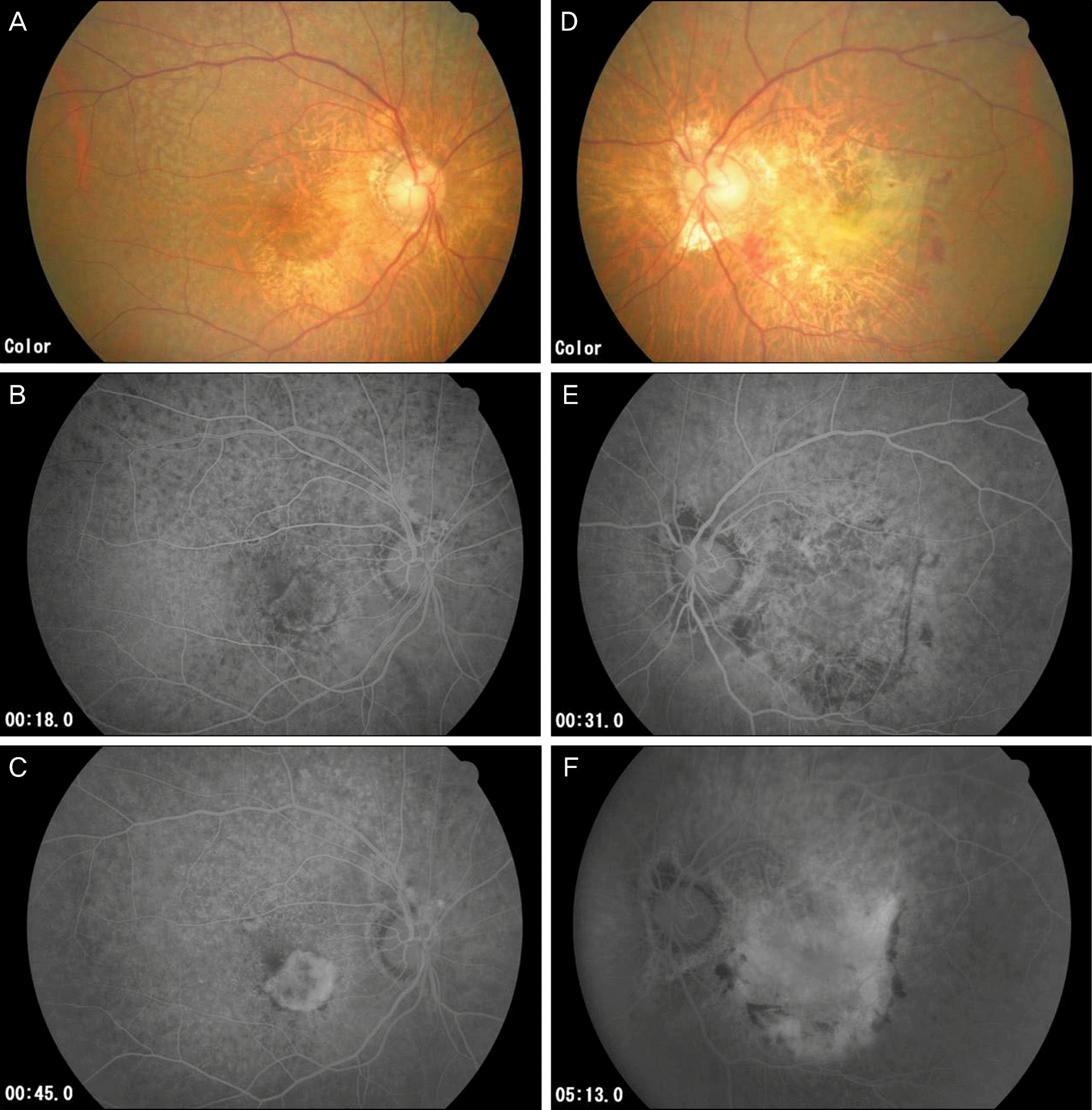
Table 1.
Baseline characteristics of patients with unilateral exudative age-related macular degeneration (AMD) at baseline
Table 2.
Number(%) of patients with fellow eyes exudative AMD conversion in each type of AMD




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download