Abstract
Purpose
In this study we compared the effectiveness between half energy photodynamic therapy (PDT) and intravitreal bevacizumab (IVB) injection for chronic central serous chorioretinopathy (CSC).
Methods
Forty-five eyes of 42 patients diagnosed as chronic CSC from March 2008 to April 2011 were treated with half energy PDT or IVB injection. The subjects were chosen for a retrospective study and analysis was performed on changes in best corrected visual acuity and existence of subretinal fluid, recurrence rate and changes in central macular thickness.
Results
Similar improvement of visual acuity was observed in both treatment groups 1 month after treatment and no meaningful difference was found in each stage for both groups (p = 0.001, p = 0.0012, respectively). However, 6 to 12 months after the treatment, the half energy PDT group showed more improvement in visual acuity compared to the IVB injection group (p = 0.019, p = 0.043, respectively). Nineteen out of 21 cases showed full recovery of subretinal fluid in the half energy PDT group with an average treatment period of 1.3 ± 0.8 months and 7 out of 24 cases showed full recovery in the IVB injection group with an average treatment period of 3.2 ± 2.8 months. There was a single case of recurrence in the half energy PDT group and 4 in the IVB injection group. The half energy PDT group showed a meaningful decline in central macular thickness at 1, 3, and 6 months after treatment (p = 0.001, p = 0.005, p = 0.007, respectively) compared to the IVB injection group and showed numerous cases with decline in central macular thickness below the 2 standard deviation from normal values (p = 0.002).
Conclusions
Both half energy PDT and IVB injection were effective for the treatment of chronic central serous chorioretinopathy. However, the half energy PDT group comparatively showed better anatomical and functional outcomes. The thinning of central macular thickness below normal value was also observed, thus careful choice of treatment is necessary for patients with chronic central serous chorioretinopathy.
Go to : 
References
1. Von Graefe A. Ueber centrale recidivirende retinitis. Albrecht von Graefes Arch Clin Exp Ophthalmol. 1866; 12:211–5.
2. Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium II. Idiopathic central serous chorioretinopathy. Am J Ophthalmol. 1967; 63:587–615.
3. Gelber GS, Schatz H. Loss of vision due to central serous chorioretinopathy following psychological stress. Am J Psychiatry. 1987; 144:46–50.
5. Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121:26–34.
6. Spaide RF, Hall L, Haas A. . Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996; 16:203–13.

7. Yap EY, Robertson DM. The long-term outcome of central serous chorioretinopathy. Arch Ophthalmol. 1996; 114:689–92.

8. Loo RH, Scott IU, Flynn HW Jr. . Factors associated with reduced visual acuity during long-term follow-up patients with idiopathic central serous chorioretinopathy. Retina. 2002; 22:19–24.
9. Leaver P, Williams C. Argon laser photocoagulation in the treat- mentof central serous retinopathy. Br J Ophthalmol. 1979; 63:674–7.
10. Robertson DM, Ilstrup D. Direct, indirect and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol. 1983; 95:457–66.

11. Piccolino FC. Laser treatment of eccentric leaks in central serous chorioretinopathy resulting in disappearance of untreated juxtafo- veal leaks. Retina. 1992; 12:96–102.
12. Schmidt-Erfurth U, Laqua H, Schlötzer-Schrehard U, Viestenz A, Naumann GO. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002; 120:835–44.
13. Lai TY, Chan WM, Lam DS. Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am J Ophthalmol. 2004; 137:826–33.

14. Schlotzer-Schrehardt U, Viestenz A, Naumann GO. . Doserelated structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002; 240:748–57.
15. Artunay O, Yuzbasioglu E, Rasier R. . Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010; 35:91–8.

16. Schaal KB, Hoeh AE, Scheuerle A. . Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009; 19:613–7.

17. Seong HK, Bae JH, Kim ES. . Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica. 2009; 223:343–7.

18. Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002; 47:431–48.

19. Guyer DR, Yannuzzi LA, Slakter JS. . Digital indocyaine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994; 112:1057–62.
20. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Arch Ophthalmol. 1999; 117:1329–45.
21. Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin: one-year results of a randomized clinical trial-VIP report 1. Ophthalmology. 2001; 108:841–52.
22. Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in Age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neo- vascularization-VIP report 2. Am J Ophthalmol. 2001; 131:541–60.
23. Chan WM, Lam DS, Lai TY. . Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003; 87:1453–8.

24. Cardillo Piccolino F, Eandi CM, Ventre L. . Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003; 23:752–63.

25. Tzekov R, Lin T, Zhang KM. . Ocular changes after photodynamic therapy. Invest Ophthalmol Vis Sci. 2006; 47:377–85.

26. Chang MH, Kim SW, Oh JR, Huh K. Photodynamic therapy with verteporfin using half fluence for chronic central serous chorioretinopathy. J Korean Ophthalmol Soc. 2009; 50:1326–33.

27. Kim JL, Kim HW, Yoon IH. Photodynamic therapy with verte- porfin for short time for chronic central serous chorioretinopathy. J Korean Ophthalmol Soc. 2008; 49:1078–86.
28. Chan WM, Lai TY, Lai RY. . Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy. One-year results of a randomized controlled trial. Ophthalmology. 2008; 115:1756–65.
29. Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010; 30:100–6.

30. Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Ophthalmol. 2011; 249:969–74.

31. Sin BH, Jeoung JK, Park SP. A case of central serous chorioretinopathy associated with retinal detachment improved by intravitreal bevacizumab injection. J Korean Ophthalmol Soc. 2010; 51:1419–22.

32. Lee JY, Chae JB, Yang SJ. . Intravitreal bevacizumab versus the conventional protocol of photodynamic therapy for treatment of chronic central serous chorioretinopathy. Acta Ophthalmol. 2011; 89:293–4.

33. Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V. . A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008; 246:1235–9.

34. Semeraro F, Romano MR, Danzi P. . Intravitreal bevacizumab versus low-fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2012; 56:608–12.

Go to : 
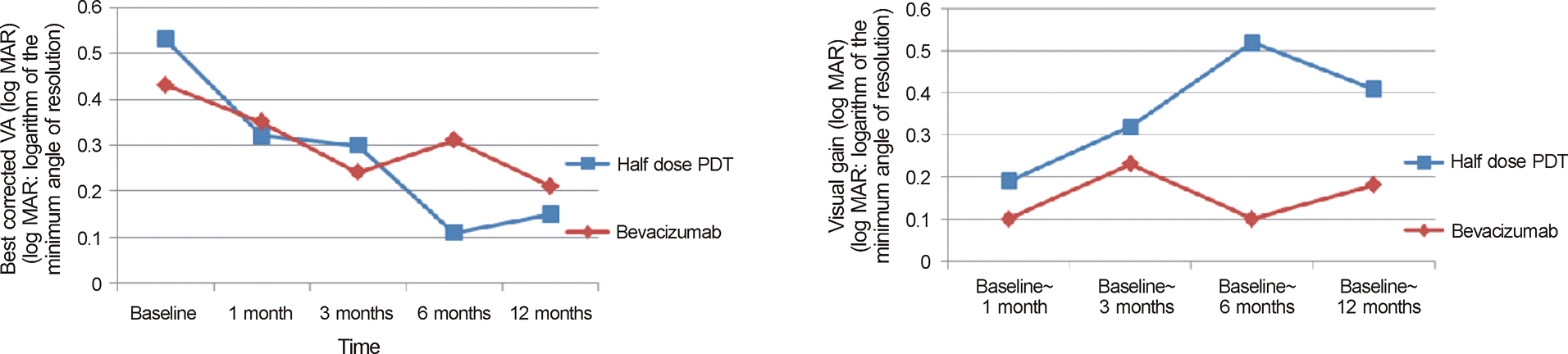 | Figure 1.Time course of mean visual acuity and visual gain during 12 months after treatment. |
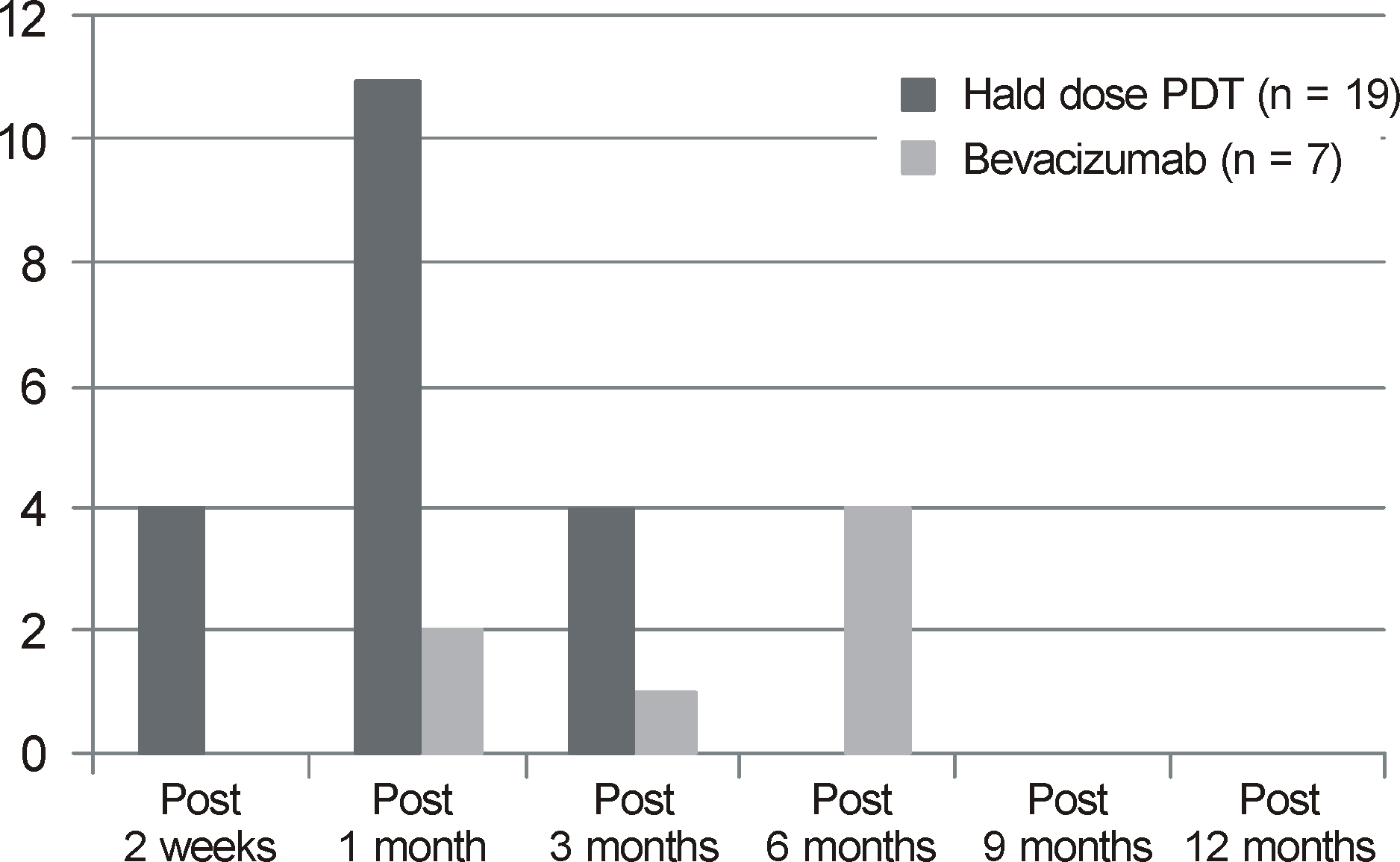 | Figure 2.Time for resolusion of subretinal fluid of patients with chronic central serous chorioretinopathy. |
Table 1.
Baseline characteristics of patients with chronic central serous chorioretinopathy
| Characteristic | hf-PDT (n = 21 eyes) | IVB (n = 24 eyes) | p-value |
|---|---|---|---|
| Sex (male:female) | 20:1 | 22:2 | 1.000† |
| Age (years) | 51.1 ± 9.1 | 51.5 ± 8.8 | 0.991* |
| Laterality (OD:OS) | 13:8 | 11:13 | 0.218† |
| Duration of chronic CSC (months) | 29.4 ± 41.6 | 25.7 ± 42.6 | 0.630* |
| Fluoroangiographic pattern | |||
| Focal leaking | 5 | 15 | |
| Diffuse, granular leaking | 16 | 9 | 0.016† |
| Follow up period (months) | 8.4 ± 7.7 | 10.0 ± 9.0 | 0.498* |
| Number of treatment | 1 | 1.9 ± 1.3 | 0.001* |
Table 2.
Clinical results of patients with chronic central serous chorioretinopathy
| BCVA (log MAR) |
hf-PDT (n = 21 eyes) |
IVB (n = 24 eyes) |
p-value† | ||||
|---|---|---|---|---|---|---|---|
| n | BCVA | Visual gain from baseline (p-value*) | n | BCVA | Visual gain from baseline (p-value*) | ||
| Baseline | 21 | 0.53 ± 0.31 | 24 | 0.43 ± 0.34 | 0.267 | ||
| Post 1 month | 21 | 0.32 ± 0.27 | 0.001 | 24 | 0.35 ± 0.35 | 0.012 | 0.854 |
| Post 3 months | 10 | 0.30 ± 0.23 | 0.011 | 16 | 0.24 ± 0.18 | 0.001 | 0.524 |
| Post 6 months | 5 | 0.11 ± 0.24 | 0.043 | 17 | 0.31 ± 0.25 | 0.091 | 0.12 |
| Post 12 months | 10 | 0.15 ± 0.22 | 0.005 | 7 | 0.21 ± 0.20 | 0.465 | 0.475 |
Table 3.
Clinical results of patients with chronic central serous chorioretinopathy
| Visual gain (log MAR) |
hf-PDT (n = 21eyes) |
IVB (n = 24 eyes) |
p-value† | ||
|---|---|---|---|---|---|
| n | Visual gain | n | Visual gain | ||
| Post 1 month ∼ Baseline | 21 | 0.19 ± 0.26 | 24 | 0.10 ± 0.18 | 0.174 |
| Post 3 months ∼ Baseline | 10 | 0.32 ± 0.31 | 16 | 0.23 ± 0.24 | 0.494 |
| Post 6 months ∼ Baseline | 5 | 0.52 ± 0.33 | 17 | 0.10 ± 0.26 | 0.019 |
| Post 12 months ∼ Baseline | 10 | 0.41 ± 0.28 | 7 | 0.18 ± 0.43 | 0.043 |
Table 4.
Subretinal fluid of patients with chronic central serous chorioretinopathy
| Subretinal fluid | hf-PDT (n = 21 eyes) | IVB (n = 24 eyes) | p-value |
|---|---|---|---|
| Location (pre) | |||
| Subfoveal, only (<200 μm) | 10 | 12 | |
| Extrafoveal (> 200 μm) | 11 | 12 | |
| Extrafoveal, only | 0 | 0 | 1.000† |
| Remission | |||
| Complete | 19 | 7 | |
| Incomplete | 2 | 17 | 0.000† |
| Period (months) | 1.3 ± 0.8 | 3.2 ± 2.8 | 0.188* |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download