Abstract
Purpose
To evaluate the effect of diabetic retinopathy on choroidal thickness and the changes of choroidal thickness after intravitreal bevacizumab injection (IVB) in diabetic patients.
Methods
The present study included 105 patients (105 eyes). Patients were classified into 6 groups: control group (A); no change (B), mild (C), moderate (D), and severe (E) non proliferative diabetic retinopathy (NPDR); and proliferative diabetic retinopathy (PDR) (F), with 15 diabetic patients in each group. All patients underwent enhanced depth imaging spectral- domain optical coherence tomography (EDI OCT) to evaluate choroidal thickness. An additional 15 patients (15 eyes) with diabetic retinopathy treated with IVB were also included. These patients underwent EDI OCT before and 1 month after IVB.
Results
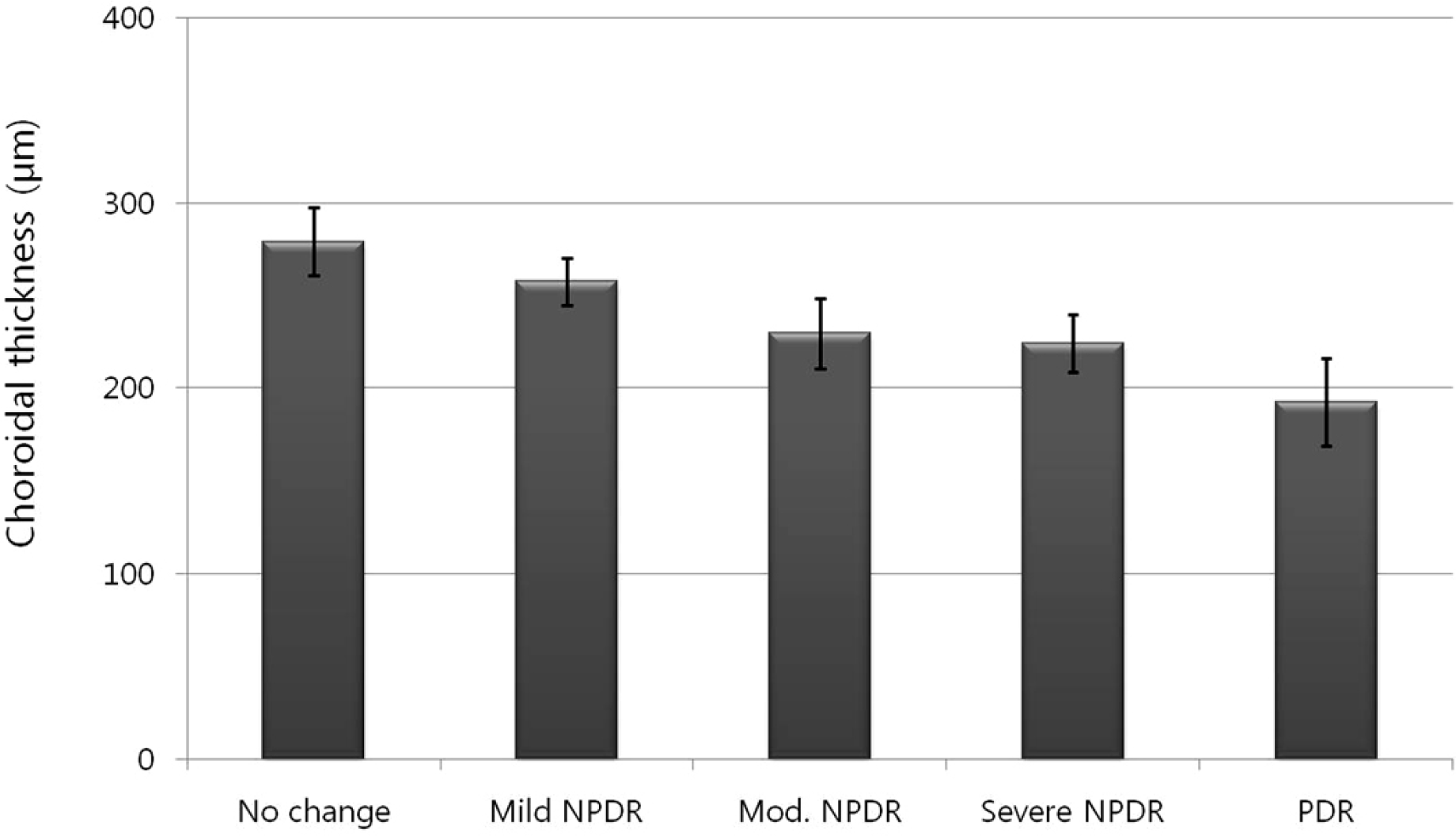
Mean choroidal thickness was significantly thinner in the moderate and severe NPDR, and PDR groups compared with the control group. Moreover, when comparing sequential stages of diabetic retinopathy progression, the choroidal thickness in the moderate NPDR stage and PDR stage was found to be significantly thinner than in the mild and severe NPDR, respectively. Additionally, choroidal thickness was 187.3 μm before IVB and significantly decreased to 168.9 μm 1 month after IVB (p < 0.05).
References
1. Seo JW, Park IW. Intravitreal bevacizumab for treatment of diabetic macular edema. Korean J Ophthalmol. 2009; 23:17–22.

2. Velez-Montoya R, Fromow-Guerra J, Burgos O. . The effect of unilateral intravitreal bevacizumab (avastin), in the treatment of diffuse bilateral diabetic macular edema: a pilot study. Retina. 2009; 29:20–6.
3. Avery RL. Regression of retinal and iris neovascularization after in- travitreal bevacizumab (Avastin) treatment. Retina. 2006; 26:352–4.
4. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous haemorrhage. Retina. 2006; 26:275–8.
5. Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998; 116:589–97.

6. Regatieri CV, Branchini L, Carmody J. . Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012; 32:563–8.

7. Querques G, Lattanzio R, Querques L. . Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012; 53:6017–24.

8. Marneros AG, Fan J, Yokoyama Y. . Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005; 167:1451–9.

9. Heiduschka P, Fietz H, Hofmeister S. . Penetration of bev-acizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007; 48:2814–23.

10. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146:496–500.

11. McLeod DS, Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994; 35:3799–811.
12. Funatsu H, Yamashita H, Sakata K. . Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005; 112:806–16.

13. Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H. . Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study. Ophthalmology. 2007; 114:743–50.
14. Aiello LP, Avery RL, Arrigg PG. . Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

16. Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptoractivation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006; 47:3135–42.
17. Chen TC, Cense B, Miller JW. . Histologic correlation of in vivo optical coherence tomography images of the human retina. Am J Ophthalmol. 2006; 141:1165–8.

18. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–5.

19. Lee SH, Chung H, Kim HC. subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. J Korean Ophthalmol Soc. 2012; 53:982–7.

20. Fujiwara T, Imamura Y, Margolis R. . Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009; 148:445–50.

22. Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010; 51:2173–6.

Figure 1.
The choroid is seen in cross-section using EDI- OCT. Subfoveal choroidal thickness was measured vertically from the outer border of the retinal pigment epithelium to the inner border of the sclera.
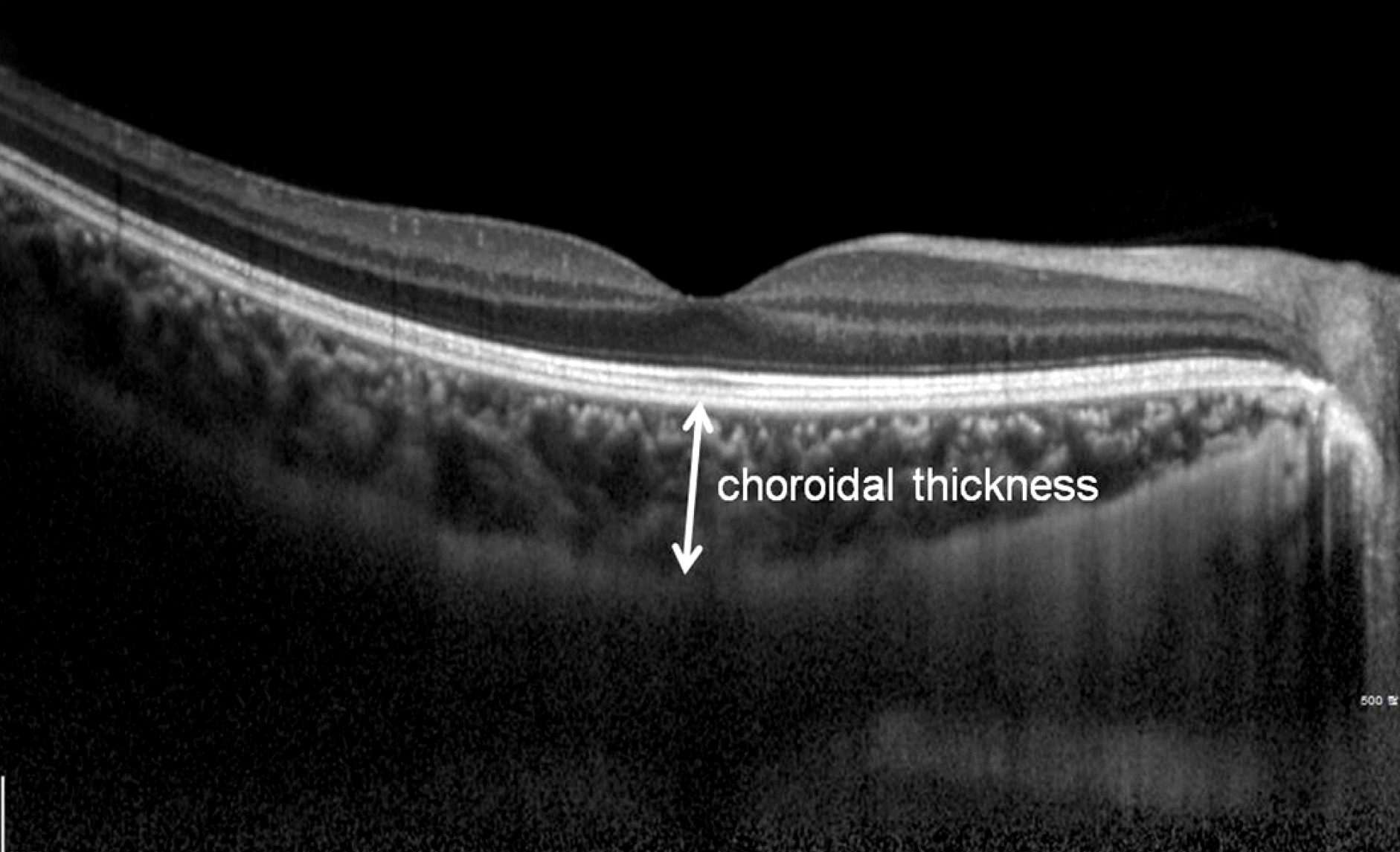
Figure 3.
Representative case of a 51-year-old man with diabetic macular edema was treated with IVB. (A) The VA was 20/30, and the subfoveal choroidal thickness was 176 μm, at initial visit. (B) At one month after first IVB, the VA was improved to 20/25. The subfoveal choroidal thickness was 164 μm.
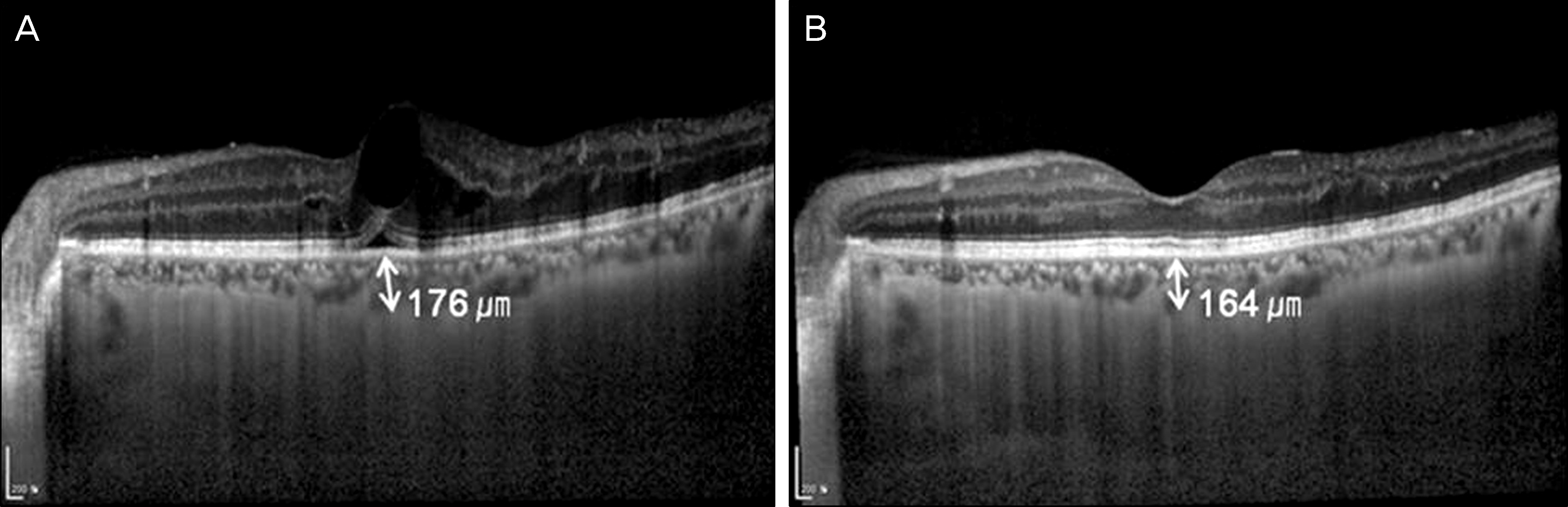
Table 1.
Characteristics of control subjects and diabetic patients
Table 2.
Comparison of choroidal thickness among stages of diabetic retinopathy progression (p-value)
| No change | Mild NPDR | Moderate NPDR | Severe NPDR | PDR | |
|---|---|---|---|---|---|
| Control | 0.512 | 0.056 | 0.002* | <0.001* | <0.001* |
| No change | − | 0.233 | − | − | − |
| Mild | − | − | 0.045* | − | − |
| Moderate | − | − | − | 0.902 | − |
| Severe | − | − | − | − | 0.026* |
Table 3.
Baseline characteristics of diabetic patient with intra- vitreal bevacizumab injection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download