Abstract
Purpose
To report the expression of decorin and TGF-β in partial myotomy of the extraocular muscle in rats.
Methods
Partial myotomy of the superior rectus muscle was performed on the right eye of 10 Sprague-Dawley rats followed by exposure of the left superior rectus muscle and a simple suture of the conjunctiva. The bilateral superior rectus muscle was obtained from all rats at 2 weeks postoperatively. The tissues were observed under light microscopy with hem-atoxylin-eosin, Masson's trichrome staining and immunohistochemistry.
Results
Histological examinations of the surgical area at 2 weeks after postoperatively showed irregularly concentrated fibrosis on light microscopy with hematoxylin-eosin and Masson's trichrome staining of the experimental eyes. Immnohistochemistry showed that expression of decorin was in the same location as TGF-β in the experimental group.
References
2. Dunlap EA. Surgery of muscle adhesions and effects of multiple operations. Br J Ophthalmol. 1974; 58:307–12.

3. Gomel V, Urman B, Gurgan T. Pathophysiology of adhesion abdominal and strategies for prevention. J Reprod Med. 1996; 41:35–41.
4. Hwang JM, Chang BL. Use of Viscoat for delayed postoperative adjustable suture strabismus surgery in rabbits. Binocul Vis Strabismus Q. 1996; 11:137–42.
5. Hwang JM, Chang BL. Delayed reattachment of extraocular abdominals in rabbits using thin polytetrafluoroethylene. Ophthalmic Surg Lasers. 1997; 28:59–64.
6. Oh SO, Chang BL, Lee J. Effect of mitomycin C on delayed abdominal in experimental strabismus surgery. Korean J Ophthalmol. 1995; 9:51–8.
7. Kim JH, Jeong SY, Jung MH, Hwang JM. Use of polyurethane with sustained release dexamethasone in delayed adjustable abdominal surgery. Br J Ophthalmol. 2004; 88:1450–4.
8. Hwang JM, Chang BL. Combined effect of Interceed and 5-fluo-rouracil on delayed adjustable strabismus surgery. Br J Ophthalmol. 1999; 83:788–91.

9. Siriwardena D, Khaw PT, King AJ, et al. Human antitransforming growth factor beta(2) monoclonal antibody–a new modulator of wound healing in trabeculectomy:a randomized placebo controlled clinical study. Ophthalmolgy. 2002; 109:427–31.
10. Border WA, Noble NA, Yamamoto T, et al. Natural inhibitor of transforming growth factor-beta protects against scarring in abdominal kidney disease. Nature. 1992; 360(6402):361–4.
11. He F, Zhang Q, Kuruba R, et al. Upregulation of decorin by FXR in vascular smooth muscle cells. Biochem Biophys Res Commun. 2008; 372:746–51.

12. Zanotti S, Negri T, Cappelletti C, et al. Decorin and biglycan abdominal is defferentially altered in several muscular dystrophies. Brain. 2005; 128(Pt 11):2546–55.
13. Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998; 17:1–19.

14. Thieszen SL, Rosenquist TH. Expression of collagens and decorin during aortic arch artery development: implications for matrix abdominal formation. Matrix Biol. 1995; 14:573–82.
15. Merle B, Malaval L, Lawler J, et al. Decorin inhibits cell attachment to thrombospondin-1 by binding to a KKTR-dependent cell adhesive site present within the N-terminal domain of thrombo-spondin-1. J Cell Biochem. 1997; 67:75–83.

16. Merle B, Durussel L, Delmas PD, Clézardin P. Decorin inhibits cell migration through a process requiring its glycosaminoglycan side chain. J Cell Biochem. 1999; 75:538–46.

17. De Luca A, Santra M, Baldi A, et al. Decorin-induced growth abdominal is associated with abdominal of p21, an inhibitor of cy-clin-dependent kinases. J Biol Chem. 1996; 271:18961–5.
18. Fischer JW, Kinsella MG, Levkau B, et al. Retroviral abdominal of decorin differentially affects the response of arterial smooth muscle cells to growth factors. Arterioscler Thromb Vasc Biol. 2001; 21:777–84.
19. Hildebrand A, Romarís M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fi-bromodulin with transforming growth factor beta. Biochem J. 1994; 302(Pt 2):527–34.
20. Kaname S, Ruoslahti E. Betaglycan has multiple binding sites for transforming growth factor-beta 1. Biochem J. 1996; 315(Pt 3):815–20.
21. Honardoust D, Varkey M, Hori K, et al. Small leucine-rich abdominal, decorin and fibromodulin, are reduced in postburn hypertrophic scar. Wound Repair Regen. 2011; 19:368–78.
22. Reich-Schupke S, Mumme A, Altmeyer P, Stuecker M. Decorin expression with stump recurrence and neovascularization after varicose vein surgery–a pilot study. Dermatol Surg. 2011; 37:480–5.

23. Grefte S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Model for muscle regeneration around fibrotic lesions in recurrent strain injuries. Med Sci Sports Exerc. 2010; 42:813–9.

24. Aärimaa V, Kääriäinen M, Vaittinen S, et al. Restroration of my-ofiber continuity after transection injury in the rat soleus. Neuromuscul Disord. 2004; 14:421–8.
25. Woo KJ, Lee KS, Choi DG, Choi MY. The effect of subabdominal injection of bevacizumab after resection of muscle in abdominal models. J Korean Ophthalmol Soc. 2010; 51:423–9.
26. Border WA, Noble NA, Yamamoto T, et al. Natural inhibitor of transforming growth factor-beta protects against scarring in abdominal kidney disease. Nature. 1992; 360:361–4.
27. Mohan RR, Tovey JC, Gupta R, et al. Decorin biology, expression, function and therapy in the cornea. Curr Mol Med. 2011; 11:110–28.
28. Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal abdominal expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996; 2:418–23.
29. Giri SN, Hyde DM, Braun RK, et al. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997; 54:1205–16.

Figure 1.
Light microscopic findings of the superior rectus muscles at 2 weeks after operation (H&E stain). (A, ×40; B, ×100) Control group. (C, ×40; D, ×100) Experimental group. The increasing density of fibrosis is observed in the experimental groups compared with control groups. SR = superior rectus.
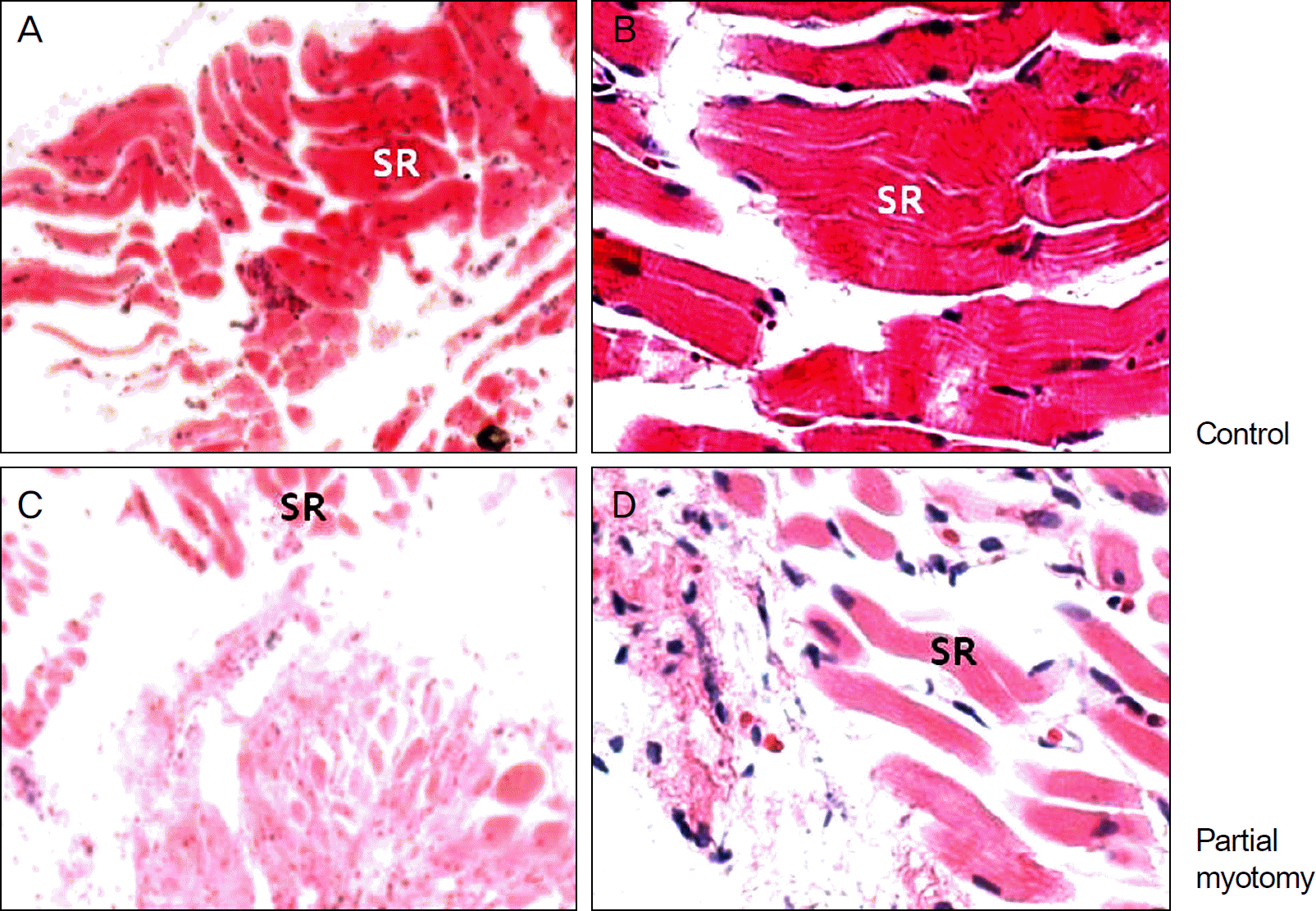
Figure 2.
Light microscopic findings of the superior rectus muscles at 2 weeks after operation (Masson's trichrome stain). (A, ×40; B, ×100) Control group. (C, ×40; D, ×100) Experimental group. The irregularity of fibrotic bands is observed more than in the experimental groups compared with control groups. SR = superior rectus.
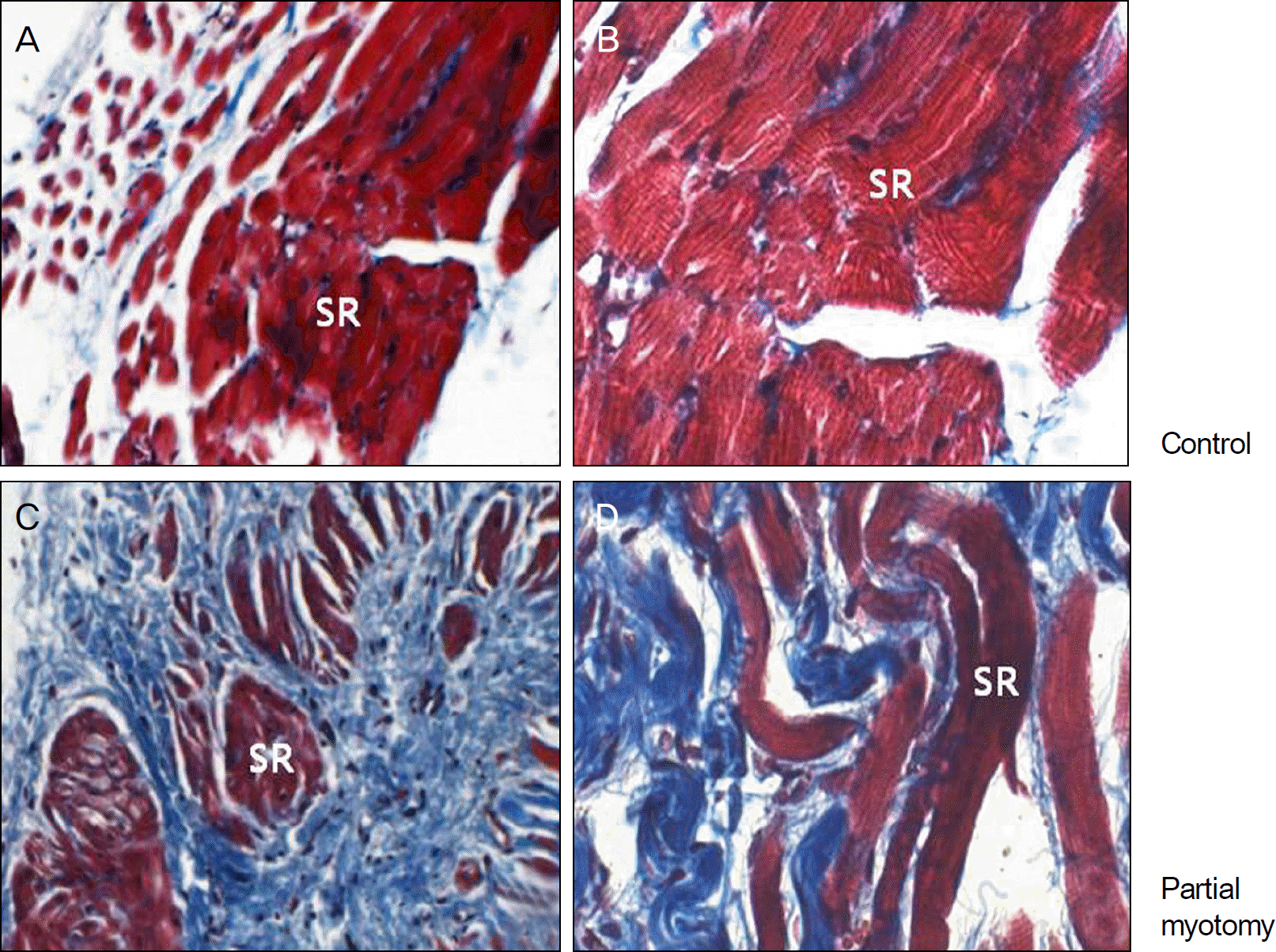
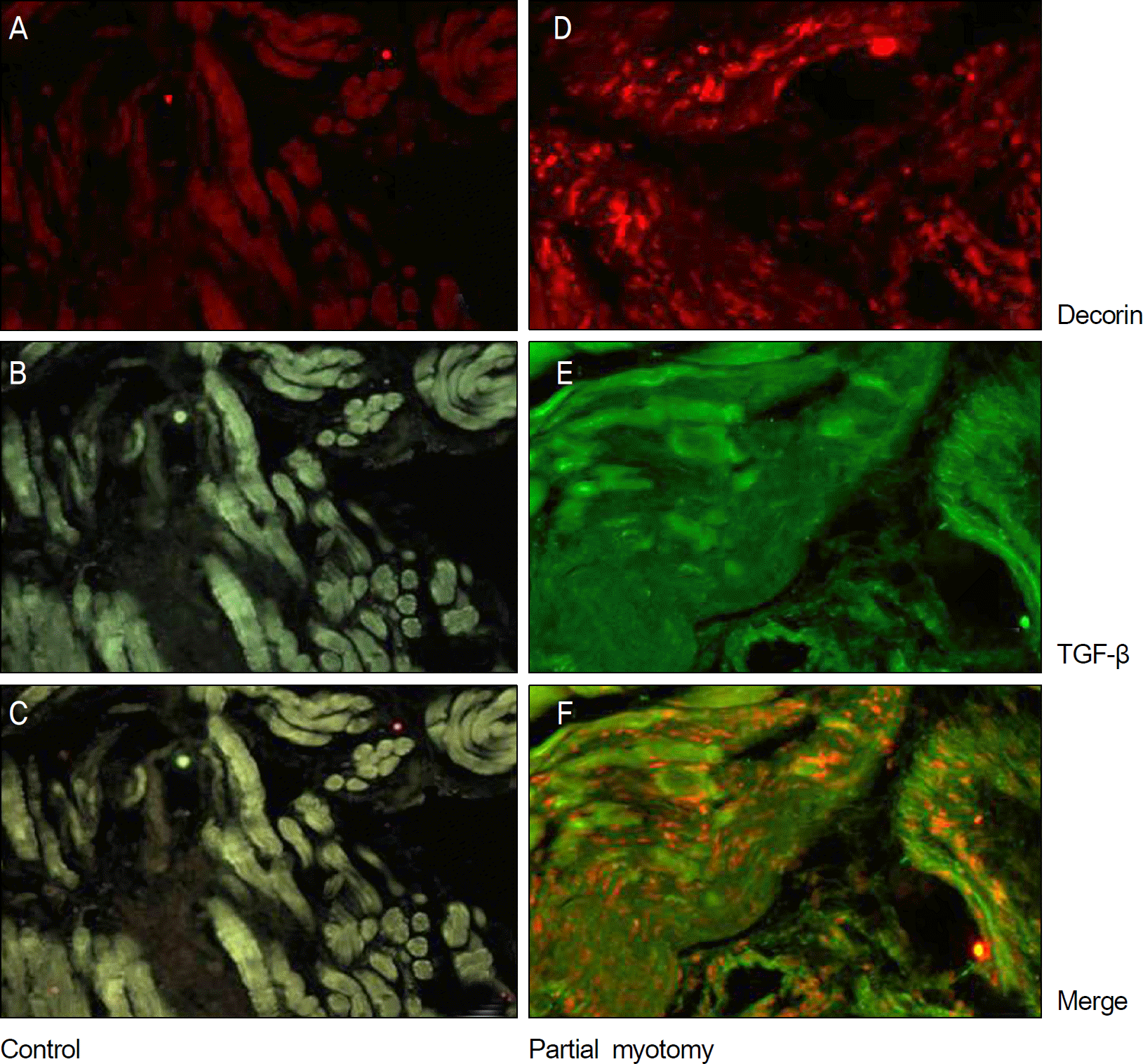
Figure 3.
The immunohistochemical staining of decorin (A, D ×200), TGF-β (B, E ×200) and merging (C, F ×200) at 2 weeks after operation. (A, B, C) No definite immune activity decorin or TGF-β were found. (D, E) The immune activity of decorin and TGF-β is observed. (F) Decorin and TGF-β are expressed at the same sites.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download