Abstract
Purpose
To report 2 cases that presented with bilateral abducens nerve palsy associated with Epstein-Barr virus (EBV) encephalitis in children.
Case summary
Case 1. A 14-month-old boy presented with fever and esodeviation of the left eye that started 5 days earlier. On the ophthalmic examination, 45-PD esotropia of the left eye and limitation of abduction in both eyes were observed. On neurological examination, there were no abnormalities. Serologic test and polymerase chain reaction (PCR) from cerebrospinal fluid (CSF) were positive for EBV. The patient was treated with systemic acyclovir and prednisolone. Part-time occlusion therapy of the right eye for 2 hours/day was also prescribed. The patient underwent a 6.5-millimeter re-cession of the medial rectus and a 6-millimeter resection of the lateral rectus on the left eye 7 months after the presentation. The patient showed orthotropia 1 week after the surgery without neurologic sequelae. Case 2. A 13-year-old boy presented with headaches and fever that started 5 days before and altered consciousness with seizures 2 days previously. Serological test for viral infection was normal, except for EBV, and CSF examination showed viral infection. After the patient recovered consciousness, he complained of diplopia. A 30-PD esotropia of his left eye with bilateral limi-tation of abduction was present. Alternating full-time occlusion of both eyes was prescribed. At 4 months after pre-sentation, diplopia disappeared and the patient showed orthotropia without abduction limitation; however, anticonvulsants were prescribed to control seizures.
Go to : 
References
1. Lee MS, Galetta SL, Volpe NJ, Liu GT. Sixth nerve palsies in children. Pediatr Neurol. 1999; 20:49–52.

2. Kodsi SR, Younge BR. Acquired oculomotor, trochlear, and abdu-cent cranial nerve palsies in pediatric patients. Am J Ophthalmol. 1992; 114:568–74.

3. Holmes JM, Mutyala S, Maus TL. . Pediatric third, fourth, and sixth nerve palsies: A population-based study. Am J Ophthalmol. 1999; 127:388–92.

4. Merino P, Gómez de Liaño P, Villalobo JM. . Etiology and treatment of pediatric sixth nerve palsy. J AAPOS. 2010; 14:502–5.

5. Patil AK, Azad ZR, Mathew V, Alexander M. Chronic meningitis and central nervous system vasculopathy related to Epstein Barr virus. Ann Indian Acad Neurol. 2012; 15:303–6.

6. Fujimoto H, Asaoka K, Imiazumi T. . Epstein-Barr virus in-fections of the central nervous system. Intern Med. 2003; 42:33–40.

7. Kalita J, Maurya PK, Kumar B, Misra UK. Epstein Barr virus ence-phalitis: clinical diversity and radiological similarity. Neurol India. 2011; 59:605–7.

8. Vetsika EK, Callan M. Infectious mononucleosis and Epstein-Barr virus. Expert Rev Mol Med. 2004; 6:1–16.

9. Doja A, Bitnun A, Jones EL. . Pediatric Epstein-Barr virus-as-sociated encephalitis:10-year review. J Child Neurol. 2006; 21:385–91.
10. Domachowske JB, Cunningham CK, Cummings DL. . Acute manifestations and neurologic sequelae of Epstein-Barr virus ence-phalitis in children. Pediatr Infect Dis J. 1996; 15:871–5.

11. Abul-Kasim K, Palm L, Maly P, Sundgren PC. The neuroanatomic localization of Epstein-Barr virus encephalitis may be a predictive factor for its clinical outcome: a case report and review of 100 cas-es in 28 reports. J Child Neurol. 2009; 24:720–6.

12. Yamashita S, Murakami C, Izumi Y. . Severe chronic active Epstein-Barr virus infection accompanied by virus-associated he-mophagocytic syndrome, cerebellar ataxia and encephalitis. Psychiatry Clin Neurosci. 1998; 52:449–52.
13. Caruso JM, Tung GA, Gascon GG. . Persistent preceding focal neurologic deficits in children with chronic Epstein-Barr virus encephalitis. J Child Neurol. 2000; 15:791–6.

14. Bray PF, Culp KW, McFarlin DE. . Demyelinating disease af-ter neurologically complicated primary Epstein-Barr virus infection. Neurology. 1992; 42:278–82.

15. Straussberg R, Cohen AH, Amir J, Varsano I. Benign abducens pal-sy associated with EBV infection. J Pediatr Ophthalmol Strabismus. 1993; 30:60.

16. Sullivan JL, Byron KS, Brewster FE. . Treatment of life threat-ening Epstein-Barr virus infection with acyclovir. Am J Med. 1982; 73:(1A). 262–6.
Go to : 
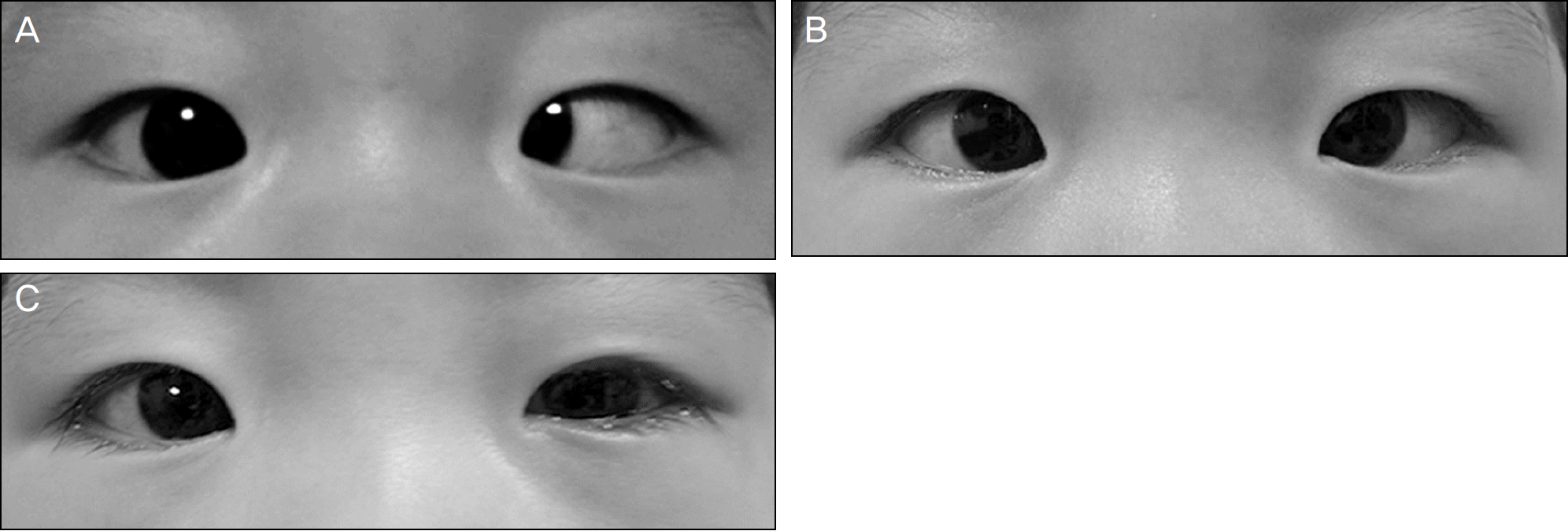 | Figure 1.In Case 1, he showed esodeviation of the left eye at primary gaze position (A). Four months later, there still was esodeviation in the left eye (B). One week after strabismus sur-gery, he showed orthotropia (C). |
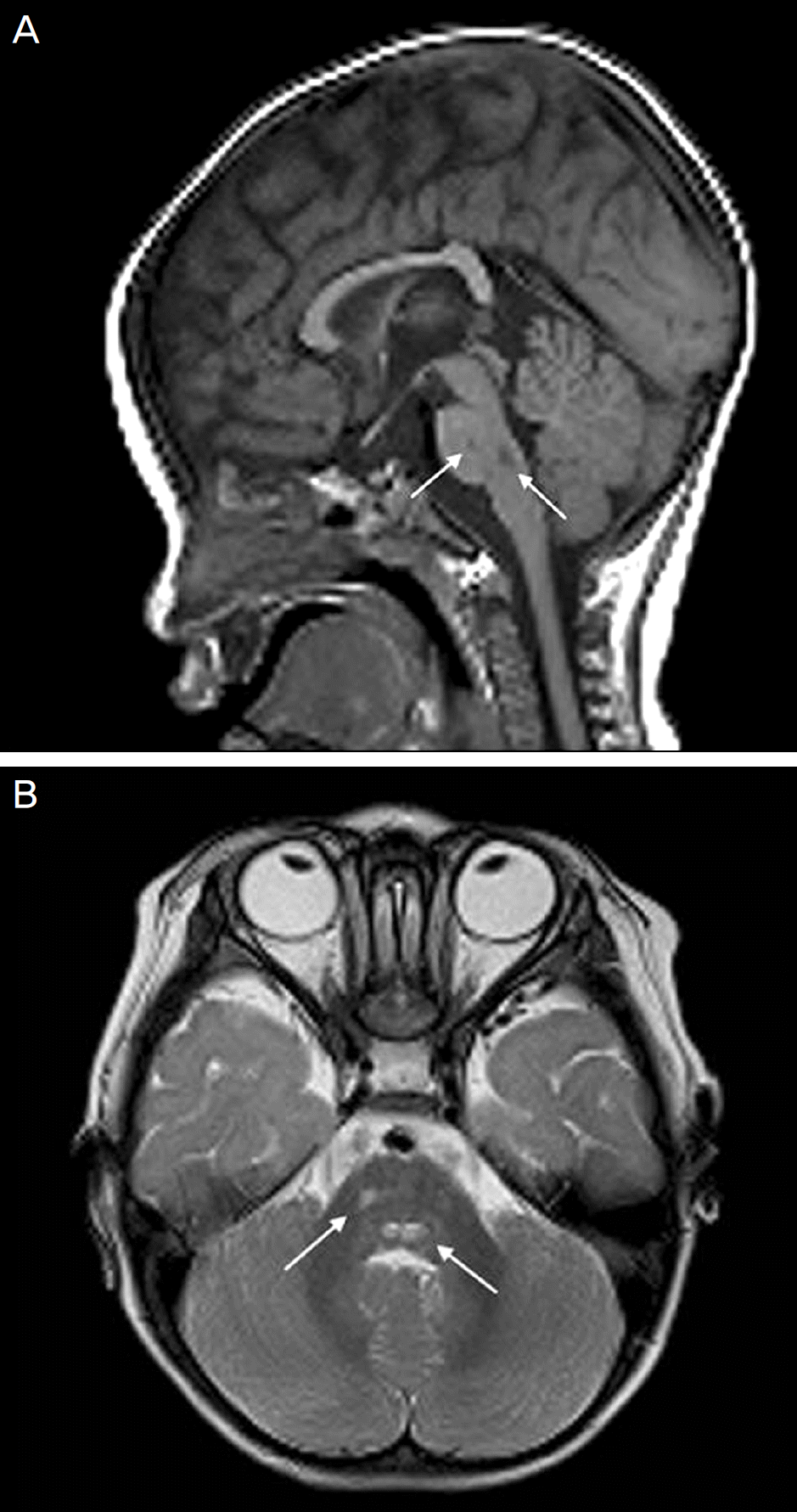 | Figure 2.Brain MRI of case 1. T1 weighted sagittal images show low signal intensity (arrows, A) and T2 weighted axial images show high signal intensity (arrows, B) without en-hancement at anterior portion of pons and medulla. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download