Abstract
Purpose
To evaluate the effect of additional retrobulbar triamcinolone acetonide (TA) injection on early recovery of visual acuity in retrobulbar optic neuritis patients.
Methods
A prospective, randomized clinical study including 30 patients with retrobulbar optic neuritis was conducted between March 2003 and June 2007. Patients were divided into 2 groups: Group 1 (n = 9) with retrobulbar triamcinolone (TA, 40 mg/1 ml) injection on the first day of ONTT protocol, and group 2 (n = 21) with conventional ONTT protocol. The following parameters were measured and analyzed: patient's sex, age, pupillary reactions, color vision, visual field, and best-corrected visual acuity before treatment, and after 1 day, 1 week, 2 weeks, 1 month, and 3 months of follow-up.
Results
Mean visual acuity before treatment was 1.00 ± 0.89 log MAR units in group 1 and 0.98 ± 0.75 log MAR units in group 2. One day after injection, visual acuity was better in group 1 (0.50 ± 0.42 log MAR units) than in group 2 (0.73 ± 0.61 log MAR units), however, there was no statistically significant difference between the 2 groups (p = 0.07). There was no significant difference in visual acuity, recovery of RAPD, color vision, or visual field at 3 months of follow-up. No serious side effect related to retrobulbar TA injection was observed.
Go to : 
References
2. Rizzo JF 3rd, Lessell S. Risk of developing multiple sclerosis after uncomplicated optic neuritis: a long-term prospective study. Neurology. 1988; 38:185–90.
3. Optic Neuritis Study Group. Multiple sclerosis risk after optic abdominal: final optic neuritis treatment trial follow-up. Arch Neurol. 2008; 65:727–32.
4. Foroozan R, Buono LM, Savino PJ, Sergott RC. Acute demyelinating optic neuritis. Curr Opin Ophthalmol. 2002; 13:375–80.

5. Lee YJ, Chang BL. Clinical manifestations of optic neuritis. J Korean Ophthalmol Soc. 1997; 38:1969–74.
7. Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, abdominalled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992; 326:581–8.
8. Optic Neuritis Study Group. Visual function 15 years after optic neuritis: a final follow-up report from the Optic Neuritis Treatment Trial. Ophthalmology. 2008; 115:1079–82.
9. Beck RW, Gal RL. Treatment of acute optic neuritis: a summary of findings from the optic neuritis treatment trial. Arch Ophthalmol. 2008; 126:994–5.
10. Volpe NJ. The optic neuritis treatment trial: a definitive answer and profound impact with unexpected results. Arch Ophthalmol. 2008; 126:996–9.
11. Keltner JL, Johnson CA, Spurr JO, Beck RW. Visual field profile of optic neuritis. One-year follow-up in the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1994; 112:946–53.

12. Lincoff HA, Kreissig I, Lincoff N, et al. Elevated concentration of intraocular cortisol obtained by sequential parabulbar injection in rabbit model. Klin Monbl Augenheilkd. 2001; 218:445–50.
13. Gould ES, Bird AC, Leaver PK, McDonald WI. Treatmenf of optic neuritis by retrobulbar injection of triamcinolone. Br Med J. 1977; 1:1495–7.

14. Feldman-Billard S, Lissak B, Kassaei R, et al. abdominal tolerance of pulse methylprednisolone therapy in patients with diabetes mellitus. Ophthalmology. 2005; 112:511–5.
15. Kim JY, Ahn M. Side effects of intravenous methylprednisolone pulse therapy in eye diseases. J Korean Ophthalmol Soc. 2008; 49:14–8.

16. Schindler RH, Chandler D, Thresher R, Machemer R. The abdominal of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982; 93:415–7.
17. Bakri SJ, Beer PM. The effect of intravitreal triamcinolone abdominal on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003; 34:386–90.
18. Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004; 122:336–40.
19. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

20. Roth DB, Chieh J, Spirn MJ, et al. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol. 2003; 121:1279–82.

21. Jonas JB, Söfker A, Degenring R. Intravitreal triamcinolone abdominal as an additional tool in pars plana vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol. 2003; 13:468–73.
Go to : 
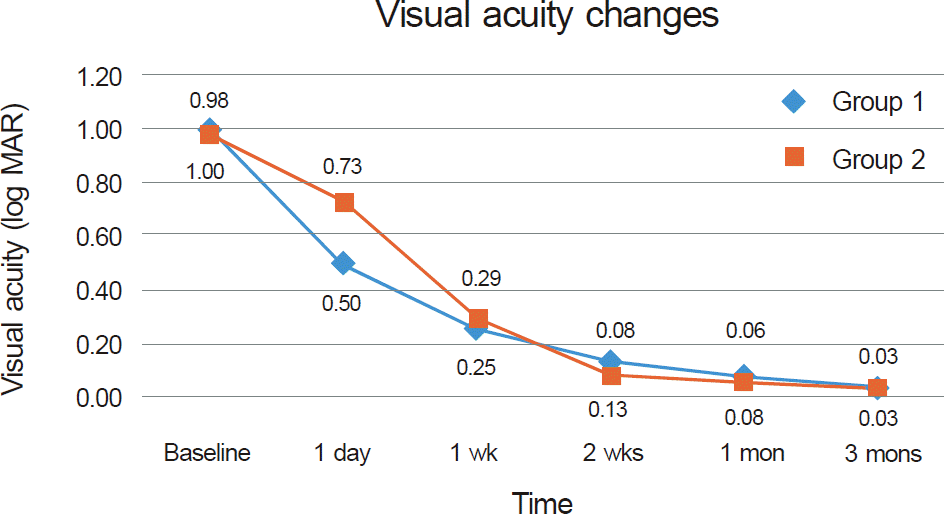 | Figure 1.Mean BCVA before treatment and after 1 day, 1 week, 2 weeks, 1 month, and 3 months of follow-up. |
Table 1.
Patient data
| Group 1 | Group 2 | p-value | |
|---|---|---|---|
| Age (yr) | 39.89 ± 14.00 | 33.14 ± 15.71 | 0.12* |
| Sex | 1.00† | ||
| Male | 3 | 6 | |
| Female | 6 | 15 | |
| Mean BCVA at diagnosis (log MAR) | 1.00 ± 0.89 | 0.98 ± 0.75 | 0.95* |
| Follow-up period (mons) | 4.52 ± 1.08 | 5.79 ± 1.42 | 0.28* |
Table 2.
Comparison of the amount of VA changes between 2 groups
| Time interval after treatment |
The amount of VA changes* (log MAR) |
||
|---|---|---|---|
| Group 1 | Group 2 | p-value† | |
| 1 day | 0.53 ± 0.58 | 0.26 ± 0.45 | 0.07 |
| 1 wk | 0.74 ± 0.64 | 0.71 ± 0.69 | 0.94 |
| 2 wks | 0.87 ± 0.76 | 0.93 ± 0.73 | 0.86 |
| 1 mon | 0.92 ± 0.81 | 0.96 ± 0.75 | 0.93 |
| 3 mons | 0.97 ± 0.88 | 0.99 ± 0.76 | 0.98 |
Table 3.
BCVA at 3 months after treatment
| BCVA at 3 mons (Snellen) | Group 1 | Group 2 | p-value |
|---|---|---|---|
| ≥20/20 | 7/9 (78) | 14/21 (67) | 0.68* |
| 20/25->20/30 | 2/9 (22) | 5/21 (24) | 1.00* |
| 20/30->20/40 | 0/9 (0) | 2/21 (9) | 1.00* |
| Mean time of regain VA of 20/20 (day) | 26.9 ± 32.1 | 33.0 ± 44.3 | 0.73† |
Table 4.
Correlations between clinical factors and BCVA at 3 months after treatment*




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download