Abstract
Purpose
To investigate the safe, effective light dose for photodynamic therapy (PDT) in the treatment of chronic central serous chorioretinopathy (CSC).
Methods
Thirty-eight eyes of 37 patients with chronic CSC were recruited for this study. From November 2009 to July 2010 and from April 2011 to February 2012, PDT was performed using 50% and 25% of the full light dose in 27 eyes of 27 patients (group I) and 11 eyes of 10 patients (group II), respectively. The minimum follow-up period was 6 months. Mean change in best corrected visual acuity (BCVA) and central retinal thickness, hyperpermeability change from abnormal cho-riocapillaris, success rate, recurrence rate, and complications were analyzed.
Results
Group I showed that BCVA (log MAR) improved significantly from 0.33 ± 0.17 to 0.14 ± 0.15 at 6 months ( p < 0.001). However, there was no significant improvement of BCVA ( p = 0.050) in group II. One eye out of 27 eyes (3.7%) in group I and 5 eyes out of 11 eyes (45.5%) in group II showed recurrence at the 6-month follow-up ( p = 0.016). After initial PDT, hyperpermeability from abnormal choriocapillaris reduced or disappeared at 95.5% in group I and 54.5% in group II at month 3 ( p = 0.016). No patient in either group experienced severe adverse events.
Go to : 
References
1. Klais CM, Ober MD, Ciardella AP, Yannuzzi LA. Central serous chorioretinopathy. Ryan SJ, Hinton DR, Schachat AP, Wilkinson P, editors. 4th ed.London: Elsevier Mosby;2006.
2. Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996; 121:26–34.
3. Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996; 16:203–13.

4. Jalkh AE, Jabbour N, Avila MP, et al. Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology. 1984; 91:1544–8.
5. Ie D, Yannuzzi LA, Spaide RF, et al. Subretinal exudative deposits in central serous chorioretinopathy. Br J Ophthalmol. 1993; 77:349–53.

6. Iida T, Yannuzzi LA, Spaide RF, et al. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003; 23:1–7. quiz 137–8.

7. Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988; 72:829–34.

8. Pikkel J, Beiran I, Ophir A, Miller B. Acetazolamide for central serous retinopathy. Ophthalmology. 2002; 109:1723–5.

9. Forooghian F, Meleth AD, Cukras C, et al. Finasteride for chronic central serous chorioretinopathy. Retina. 2011; 31:766–71.

10. Battaglia Parodi M, Da Pozzo S, Ravalico G. Photodynamic therapy in chronic central serous chorioretinopathy. Retina. 2003; 23:235–7.

11. Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003; 23:752–63.

12. Yannuzzi LA, Slakter JS, Gross NE, et al. Indocyanine green angiography- guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003; 23:288–98.
13. Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003; 87:1453–8.

14. Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006; 26.

15. Schltzer-Schrehardt U, Viestenz A, Naumann GO, et al. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002; 240:748–57.
16. Chan WM, Lai TY, Lai RY, et al. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008; 28:85–93.
17. Senturk F, Karacorlu M, Ozdemir H, et al. Microperimetric changes after photodynamic therapy for central serous chorioretinopathy. Am J Ophthalmol. 2011; 151:303–9.e1.

18. Michels S, Hansmann F, Geitzenauer W, Schmidt-Erfurth U. Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci. 2006; 47:371–6.

19. Zhao MW, Zhou P, Xiao HX, et al. Photodynamic therapy for acute central serous chorioretinopathy: the safe effective lowest dose of verteporfin. Retina. 2009; 29:1155–61.
20. Kaiser PK. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1132–42.
21. Arnold JJ, Blinder KJ, Bressler NM, et al. Acute severe visual acuity decrease after photodynamic therapy with verteporfin: case reports from randomized clinical trials-TAP and VIP report no. 3. Am J Ophthalmol. 2004; 137:683–96.
22. Schmidt MH, Reichert KW 2nd, Ozker K, et al. Preclinical evaluation of benzoporphyrin derivative combined with a light-emitting diode array for photodynamic therapy of brain tumors. Pediatr Neurosurg. 1999; 30:225–31.

23. Miller JW, Schmidt-Erfurth U, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol. 1999; 117:1161–73.
24. Bandello F, Virgili G, Lanzetta P, et al. [ICG angiography and retinal pigment epithelial decompensation (CRSC and epitheliopathy)]. J Fr Ophtalmol. 2001; 24:448–51.
25. Lai TY, Chan WM, Lam DS. Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am J Ophthalmol. 2004; 137:826–33.

26. Lai TY, Chan WM, Li H, et al. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006; 90:869–74.

27. Tzekov R, Lin T, Zhang KM, et al. Ocular changes after photodynamic therapy. Invest Ophthalmol Vis Sci. 2006; 47:377–85.

28. Kim M. The result of photodynamic therapy in chronic central serous chorioretinopathy. J Korean Ophthalmol Soc. 2009; 50:1035–43.

29. Song MH, Lee PY, Kim KS, Lee WK. The effect of photodynamic therapy in chronic central serous chorioretinopathy. J Korean Ophthalmol Soc. 2007; 48:1048–58.

30. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996; 103:2070–9. discussion 2079–80.

31. Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984; 91:1554–72.

32. Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986; 84:799–845.

33. Fujita K, Yuzawa M, Mori R. Retinal sensitivity after photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy: short-term results. Retina. 2011; 31:772–8.
34. Lim JW, Kang SW, Kim YT, et al. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. Br J Ophthalmol. 2011; 95:514–7.

35. Butler AL, Fung AT, Merkur AB, et al. Very minimal fluence photodynamic therapy for chronic central serous chorioretinopathy. Can J Ophthalmol. 2012; 47:42–4.

36. Uetani R, Ito Y, Oiwa K, et al. Half-dose vs one-third-dose photodynamic therapy for chronic central serous chorioretinopathy. Eye (Lond). 2012; 26:640–9.

37. Chang MH, Kim SW, Oh JR, et al. Photodynamic therapy with verteporfin using half fluence for chronic central serous chorioretinopathy. J Korean Ophthalmol Soc. 2009; 50:1326–33.

38. Chan WM, Lai TY, Lai RY, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008; 115:1756–65.
39. Feigl B, Brown B, Lovie-Kitchin J, Lee L. Dynamics of retinal function after multiple photodynamic therapies in age-related macular degeneration: a report of cases. Doc Ophthalmol. 2005; 111:135–48.

40. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials-- TAP report. Arch Ophthalmol. 1999; 117:1329–45.
41. Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-- verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001; 131:541–60.
Go to : 
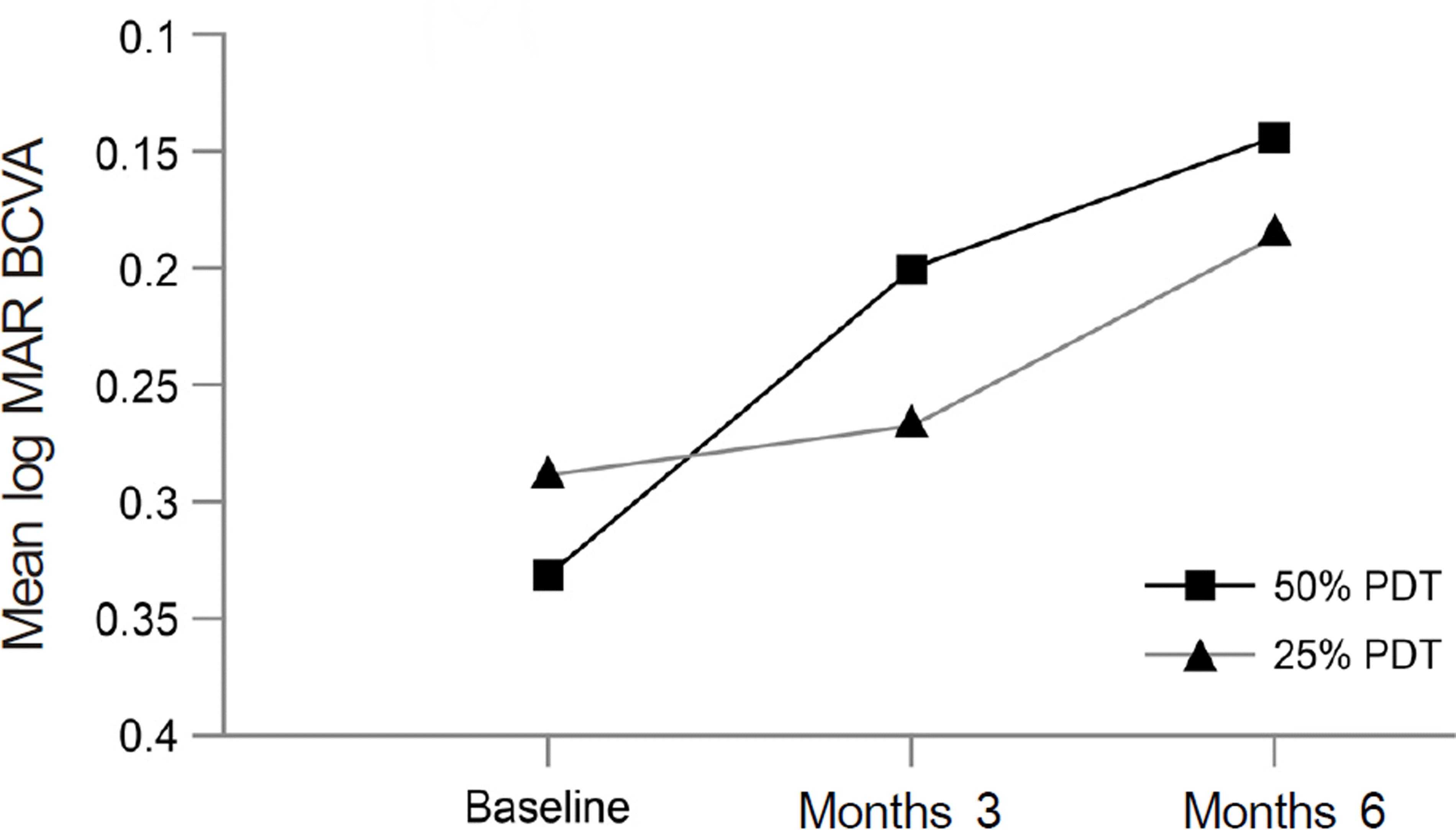 | Figure 1.Graph showing the serial changes in mean BCVA (best-corrected visual acuity) of patients in the 50% PDT group and in the 25% PDT group. While 50% PDT group showed significant visual improvement at month 3 and month 6 after treatment, 25% PDT group showed no significant visual improvement. And there was no significant difference in mean change of BCVA at every follow-up period between two groups. |
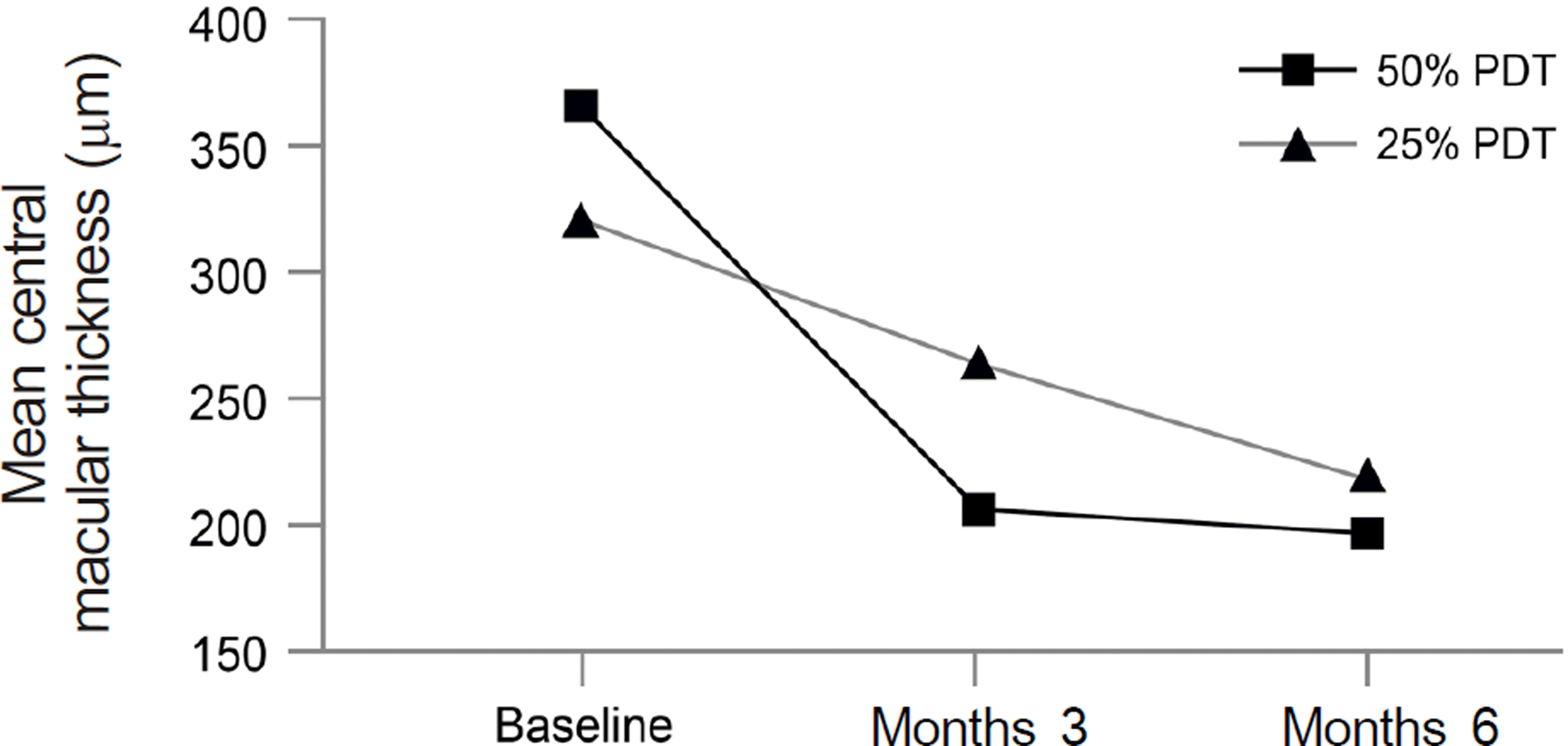 | Figure 2.Graph showing the serial changes in mean CMT (central macular thickness) in the 50% PDT group and in the 25% PDT group. 25% PDT group showed no significant decrease in CMT at month 3 after treatment but had significance at month 6. Meanwhile, 50% PDT group showed significance at every follow-up period. There was no significant difference in mean change of CMT at every follow-up period between two groups. |
Table 2.
Clinical data in patients with CSC treated by using 25% energy PDT
| Groups | 50% PDT* | 25% PDT† | p-value‡ |
|---|---|---|---|
| Baseline characteristics | |||
| Participants (eyes) | 27 | 11 | |
| Sex (M : F) | 22:5 | 7:4 | |
| Age (years) | 47.5 7.7 | 51.18 8.13 | 0.116‡ |
| Duration of symptoms (months) | 11.7 8.3 | 17.27 19.74 | 0.169‡ |
| BCVA (log MAR) | 0.33 0.17 | 0.31 0.38 | 0.124‡ |
| CMT (m) | 352.89 82.55 | 350.18 102.90 | 0.975‡ |
Table 2.
Clinical data in patients with CSC treated by using 25% energy PDT




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download