Abstract
Purpose
To investigate the clinical results, recurrence rates, and recurrence-related risk factors of temporary amniotic membrane patch (TAMP) after excision of primary pterygium.
Methods
Recurrence grade was evaluated for 73 eyes with a mean follow-up of 15.5 months (range 9 to 56 months). Surgical results were classified into surgical success (G0/G1), conjunctival recurrence (G2), and corneal recurrence (G3). Recurrence rates were analyzed based on gender, age, Tan's preoperative grading system, horizontal and vertical length of the preoperative pterygium, the corneal involvement size of the preoperative pterygium, planned or unplanned removal of amniotic membrane, and epithelial healing time. Using Kaplan-Meier survival analysis, the cumulative proportion of integrated G0/G1 was evaluated.
Results
In the postoperative grading, 58 (79.5%) and 9 (12.3%) eyes were graded as clinically recurrence-free G0 and G1, respectively; 4 (5.5%) and 2 (2.7%) eyes were graded as clinically recurrence-occurred G2 and G3, respectively. The cumulative proportion of integrated recurrence-free G0/G1 at 24 months after surgery was 0.83 ± 0.08. T3 of Tan's preoperative grading system was identified as the only risk factor for recurrence above G1 through multivariate logistic regression analysis (p = 0.02).
Conclusions
The recurrence rate of the present TAMP study showed better results in comparison with 9.1 to 56.1% of recurrence rates in other studies. The TAMP has advantages of surgical procedure with ease, low complications, and low recurrence rates. Therefore, after surgical excision of primary pterygium, the authors concluded the TAMP is highly recommended for good clinical outcomes and low recurrence rates.
Figures and Tables
 | Figure 1Preoperative grades for primary pterygium before surgery. T1 (atrophic pterygium): Episcleral vessels unobscured. T2 (intermediate pterygium): Episcleral vessels partially obscured. T3 (fleshy pterygium): Episcleral vessels totally obscured. |
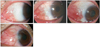 | Figure 2Postoperative grades after excision of primary pterygium. G0 (grade 0) indicates a normal appearance of the operated site. G1 (grade 1) indicates the presence of some fine episcleral vessels, but without any fibrous tissue in the excised area extending up to but not beyond the limbus. G2 (grade 2, conjunctival recurrence) indicates the presence of additional fibrous tissues in the excised area without invading the cornea. G3 (grade 3, corneal recurrence) represents a true recurrence with a fibrovascular tissue invading the cornea. |
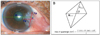 | Figure 3Pterygium size on M-view system with a mathematical formula of quadrangle. (A) Vp = vertical distance (pixel); Hp = horizontal distance (pixel); θ = angle between V & H; D = corneal diameter (mm). (B) A mathematical formula of quadrangle. |
 | Figure 4Surgical procedures of pterygium excision and temporary amniotic membrane patch. (A) The pterygium body and head is undermined and removed by blunt dissection. (B) The corneal defect area is shaved of any residual tissue with a Beaver blade and a diamond burr. (C) At the end of tissue excision, the amniotic membrane is removed from the filter paper, and placed over the conjunctival defect with the basement membrane side down. And the amniotic membrane is secured to the episclera and the excision edges of the normal conjunctiva with a continuous 10-0 nylon suture. |
 | Figure 5Incidence of each postoperative pterygium grades based on the preoperative pterygium grades. No postoperative recurrence was observed in preoperative T1 group. But, there were clinically significant G2 and G3 recurrence-occurred eyes in T2 and T3 groups; 2 eyes (4.8%; 2/42 eyes) of G3 recurrence were observed in T2 group; 4 eyes (13.8%; 4/29 eyes) of G2 recurrence were observed in T3 group. |
 | Figure 6Kaplan-Meier survival analysis showing the cumulative proportion without recurrence following the temporary amniotic membrane patch with the excision of primary pterygium. This survival curve indicates that the estimated recurrence-free (G0 & G1) rate at postoperative 24 months is 0.83 ± 0.08. |
Table 5
Risk factors influencing a recurrence of pterygium after surgery in relation to preoperative parameter

Table 6
Recurrence rates of amniotic membrane transplantation after excision of primary pterygium in recent reports

AMT = amniotic membrane transplantation; BM = basement membrane.
*Final appearance was graded according to the grading system (G0 to G3) reported by Prabhasawat et al,9 and overall recurrence rate was defined as G2 and G3; †Intraoperatively, local triamcinolone acetonide injections (10 to 16 mg in total) were given along the edges of the excised conjunctiva at the end of tissue excision; ‡The recurrence rate of the study was evaluated at postoperative 6 months.
References
1. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001. 108:449–460.
2. Verma N, Garap JA, Maris R, Kerek A. Intraoperative use of mitomycin C in the treatment of recurrent pterygium. P N G Med J. 1998. 41:37–42.
3. Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997. 115:1235–1240.
4. Riordan-Eva P, Kielhorn I, Ficker LA, et al. Conjunctival autografting in the surgical management of pterygium. Eye (Lond). 1993. 7(Pt 5):634–638.
5. Panda A, Das GK, Tuli SW, Kumar A. Randomized trial of intraoperative mitomycin C in surgery for pterygium. Am J Ophthalmol. 1998. 125:59–63.
6. Chen PP, Ariyasu RG, Kaza V, et al. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol. 1995. 120:151–160.
7. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.
8. Ye J, Kook KH, Yao K. Temporary amniotic membrane patch for the treatment of primary pterygium: mechanisms of reducing the recurrence rate. Graefes Arch Clin Exp Ophthalmol. 2006. 244:583–588.
9. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997. 104:974–985.
10. Luanratanakorn P, Ratanapakorn T, Suwan-Apichon O, Chuck RS. Randomised controlled study of conjunctival autograft versus amniotic membrane graft in pterygium excision. Br J Ophthalmol. 2006. 90:1476–1480.
11. Küçükerdönmez C, Akova YA, Altinörs DD. Comparison of conjunctival autograft with amniotic membrane transplantation for pterygium surgery: surgical and cosmetic outcome. Cornea. 2007. 26:407–413.
12. Park WC. New strategy of ocular surface disease - Ocular surface reconstruction using amniotic membrane and limbal stem cell transplantation. J Korean Med Assoc. 2005. 48:628–633.
13. Kwak DY, Lee JK, Park DJ. Pterygium surgery: Wide excision with amniotic membrane transplantation using fibrin glue. J Korean Ophthalmol Soc. 2008. 49:213–221.
14. Kobayashi A, Shirao Y, Yoshita T, et al. Temporary amniotic membrane patching for acute chemical burns. Eye (Lond). 2003. 17:149–158.
15. Kheirkhah A, Johnson DA, Paranjpe DR, et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008. 126:1059–1066.
16. Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999. 128:280–287.
17. Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001. 119:695–706.
18. Weinstein O, Rosenthal G, Zirkin H, et al. Overexpression of p53 tumor suppressor gene in pterygia. Eye (Lond). 2002. 16:619–621.
19. Tan DT, Lim AS, Goh HS, Smith DR. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997. 123:404–405.
20. Dushku N, Reid TW. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997. 16:1179–1192.
21. Shimmura S, Ishioka M, Hanada K, et al. Telomerase activity and p53 expression in pterygia. Invest Ophthalmol Vis Sci. 2000. 41:1364–1369.
22. Detorakis ET, Spandidos DA. Pathogenetic mechanisms and treatment options for ophthalmic pterygium: trends and perspectives (Review). Int J Mol Med. 2009. 23:439–447.
23. Detorakis ET, Zaravinos A, Spandidos DA. Growth factor expression in ophthalmic pterygia and normal conjunctiva. Int J Mol Med. 2010. 25:513–516.
24. Lee SB, Li DQ, Tan DT, et al. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000. 20:325–334.
25. Ti SE, Tseng SC. Management of primary and recurrent pterygium using amniotic membrane transplantation. Curr Opin Ophthalmol. 2002. 13:204–212.
26. Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005. 24:141–150.
27. Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology. 2011. 118:908–914.
28. Hayashi H, Sakai J, Matsuura H, Fujimoto W. Cyclophosphamide and amniotic membrane transplantation in the management of ocular disease in a case of antiepiligrin cicatricial pemphigoid. Clin Exp Dermatol. 2009. 34:e477–e479.
29. Zhong Y, Zhai Z, Zhou Y, et al. [Effect of amniotic membrane on expressions of TGF-beta 1, collagens I, III and fibronectin in rabbit corneal healing after photorefractive keratectomy]. Yan Ke Xue Bao. 2000. 16:239–242.
30. Shimazaki J, Kosaka K, Shimmura S, Tsubota K. Amniotic membrane transplantation with conjunctival autograft for recurrent pterygium. Ophthalmology. 2003. 110:119–124.
31. Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002. 109:1752–1755.
32. Kwak DY, Bae MC, Lee JK, Park DJ. Wide excision with conjunctivo-limbal autograft. J Korean Ophthalmol Soc. 2008. 49:205–212.
33. Mahar PS, Nwokora GE. Role of mitomycin C in pterygium surgery. Br J Ophthalmol. 1993. 77:433–435.
34. Nakamura T, Inatomi T, Sekiyama E, et al. Novel clinical application of sterilized, freeze-dried amniotic membrane to treat patients with pterygium. Acta Ophthalmol Scand. 2006. 84:401–405.
35. Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996. 93:3509–3513.
36. Mutlu FM, Sobaci G, Tatar T, Yildirim E. A comparative study of recurrent pterygium surgery: limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology. 1999. 106:817–821.
37. Ti SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol. 2000. 84:385–389.
38. Kenyon KR, Wagoner MD, Hettinger ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985. 92:1461–1470.
39. Fernandes M, Sangwan VS, Bansal AK, et al. Outcome of pterygium surgery: analysis over 14 years. Eye (Lond). 2005. 19:1182–1190.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download