Abstract
Purpose
To report a case of subconjunctival foreign body migration in both eyes after collagen-containing filler injection.
Case summary
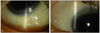
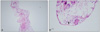
A 51-year-old female, who had been treated with collagen-containing filler in her eyelid, nose, and forehead for cosmetic complaints four months earlier, presented to our clinic with decreased visual acuity and foreign body sensation in both eyes. Slit lamp examination revealed moderate nucleosclerosis and subcapsular opacity in her crystalline lens, in addition to scattered subconjunctival foreign body infiltration in both eyes. Cataract extraction with posterior chamber lens implantation was performed, and the subconjunctival foreign body was also partially removed. Biopsy of the remaining foreign body was performed, and examination revealed foreign material and multivacuolated cells in the conjunctiva.
Figures and Tables
References
1. Hoffmann C, Schuller-Petrovic S, Soyer HP, Kerl H. Adverse reactions after cosmetic lip augmentation with permanent biologically inert implant materials. J Am Acad Dermatol. 1999. 40:100–102.
2. Alcalay J, Alkalay R, Gat A, Yorav S. Late-onset granulomatous reaction to Artecoll. Dermatol Surg. 2003. 29:859–862.
3. Engelman DE, Bloom B, Goldberg DJ. Dermal fillers: complications and informed consent. J Cosmet Laser Ther. 2005. 7:29–32.
4. Ellis DA, Makdessian AS, Brown DJ. Survey of future injectables. Facial Plast Surg Clin North Am. 2001. 9:405–411.
5. Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substance for soft tissue augmentation. Aesthetic Plast Surg. 2003. 27:354–366.
6. Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005. 31:1616–1625.
7. Danesh-Meyer HV, Savino PJ, Sergott RC. Case reports and small case series: ocular and cerebral ischemia following facial injection of autologous fat. Arch Ophthalmol. 2001. 119:777–778.
8. Lemperle G, Romano JJ, Busso M. Soft tissue augmentation with artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg. 2003. 29:573–587.
9. Jo YJ, Lee DG, Lee SB. Late onset migrated inflammatory gran uloma after collagen-containing filler injection. J Korean Ophthalmol Soc. 2008. 49:1330–1334.
10. Jung BY, Kim YD. Orbital dermoid cysts presenting as subconjunctival fat droplets. Ophthal Plast Reconstr Surg. 2008. 24:327–329.
11. Heltzer JM, Ellis DS, Stewart WB, Spencer WH. Diffuse nodular eyelid lipogranuloma following sutureless transconjunctival blepharoplasty dressed with topical ointment. Ophthal Plast Reconstr Surg. 1999. 15:438–441.
12. Fenton S, Canninga-van Dijk MR, Mourits MP. Lipogranuloma of the nasolacrimal system, an iatrogenic and preventable entity. Eye (Lond). 2003. 17:528–530.
13. Doh SH, Lee SK, Yang SW. A case of primary lipogranuloma in eyelid. J Korean Ophthalmol Soc. 2008. 49:2001–2005.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download