Abstract
Purpose
To evaluate the possible causes of decreased central corneal endothelial cell count in glaucoma patients and to acknowledge the importance of central corneal endothelial cell count evaluation.
Methods
This retrospective case-control study included 60 glaucomatous eyes that were being treated with topical anti-glaucoma drugs, 30 eyes that underwent laser iridotomy or trabeculectomy, and 60 control eyes. Intraocular pressure, duration of topical anti-glaucoma medications, mean number of topical anti-glaucoma drugs, and preoperative central corneal endothelial cells were analyzed.
Results
The numbers of central corneal endothelial cells in the topical anti-glaucoma medication group, the surgical group and the control group were 2681.30 ± 355.33, 2435.57 ± 646.81 and 2822.08 ± 330.17 cells/mm2, respectively. The numbers in the topical anti-glaucoma medication group and the surgical group were significantly lower than that of the control group. The number of central corneal endothelial cells was significantly low in patients with longer duration of disease and in those taking a greater number of topical anti-glaucoma drugs (p = 0.003, p = 0.010)
Figures and Tables
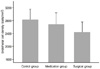 | Figure 1Comparison of the endothelial cell density of the control groups (n = 60), topical anti-glaucoma medication group (n = 60) and surgical group (n = 30). |
 | Figure 2Correlation between the corneal endothelial cell density and Intraocular pressure in topical anti-glaucoma medication group (r = 0.252, p = 0.062) |
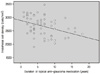 | Figure 3Correlation between the corneal endothelial cell density and duration of topical anti-glaucoma medication in topical anti-glaucoma medication group (r = 0.378, p = 0.003). |
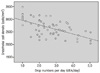 | Figure 4Correlation between the corneal endothelial cell density and drop numberzs per a day in topical anti-glaucoma medication group (r = 0.604, p = 0.010). Drop number (dEA) is defined as a total number of drops actually instilled into the eye. |
Table 2
Comparison of control group and surgical treated group

Values are presented as mean ± SD unless otherwise indicated.
CV = coefficient of variation of the cell area; CD = corneal endothelial cell density; LI = laser iridotomy.
*p-value was determined by unpaired t-test compared with control group; †p-value was determined by Mann Whitney U-test compared with control group.
References
1. Shaffer RN, Rosenthal G. Comparison of cataract incidence in normal and glaucomatous population. Am J Ophthalmol. 1970. 69:368–371.
2. Sugar J, Mitchelson J, Kraff M. Endothelial trauma and cell loss from intraocular lens insertion. Arch Ophthalmol. 1978. 96:449–450.
3. Sugar J, Mitchelson J, Kraff M. The effect of phacoemulsification on corneal endothelial cell density. Arch Ophthalmol. 1978. 96:446–448.
4. Na HK, Lee SW. Change of the corneal endothelial cell density in postoperative. J Korean Ophthalmol Soc. 1981. 22:17–24.
5. Gwin RM, Warren JK, Samuelson DA, Gum GG. Effects of phacoemulsification and extracapsular lens removal on corneal thickness and endothelial cell density in the dog. Invest Ophthalmol Vis Sci. 1983. 24:227–236.
6. Jacobi PC, Dietlein TS, Luke C, et al. Primary phacoemulsification and intraocular lens implantation for acute angle-closure glaucoma. Ophthalmology. 2002. 109:1597–1603.
7. Khokhar S, Sindhu N, Pangtey MS. Phacoemulsification in filtered chronic angle closure glaucoma eyes. Clin Experiment Ophthalmol. 2002. 30:256–260.
8. Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and co-existing cataract: a prospective case series. J Glaucoma. 2006. 15:47–52.
9. Itoi M, Nakamura T, Mizobe K, et al. Specular microscopic studies of the corneal endothelia of Japanese diabetics. Cornea. 1989. 8:2–6.
10. Larsson LI, Bourne WM, Pach JM, Brubaker RF. Structure and function of the corneal endothelium in diabetes mellitus type I and type II. Arch Ophthalmol. 1996. 114:9–14.
11. Lass JH, Spurney RV, Dutt RM, et al. A morphologic and fluorophotometric analysis of the corneal endothelium in type I diabetes mellitus and cystic fibrosis. Am J Ophthalmol. 1985. 100:783–788.
12. Schultz RO, Matsuda M, Yee RW, et al. Corneal endothelial changes in type I and type II diabetes mellitus. Am J Ophthalmol. 1984. 98:401–410.
13. Straatsma BR, Pettit TH, Wheeler N, Miyamasu W. Diabetes mellitus and intraocular lens implantation. Ophthalmology. 1983. 90:336–343.
14. Whikehart DR, Montgomery B, Angelos P, Sorna D. Alteration of ATPase activity and duplex DNA in corneal cells grown in high glucose media. Cornea. 1993. 12:295–298.
15. Kim KS, Park SY, Oh JS. Morphometric analysis of the corneal endothelial cells in normal Korean. J Korean Ophthalmol Soc. 1992. 33:320–325.
16. Markowitz SN, Morin JD. The endothelium in primary angle-closure glaucoma. Am J Ophthalmol. 1984. 98:103–104.
17. Waltman SR, Yarian D, Hart W Jr, Becker B. Corneal endothelial changes with long-term topical epinephrine therapy. Arch Ophthalmol. 1977. 95:1357–1358.
18. Koo BS, Chung J, Baek NH. The effect of extracapsular cataract extraction in patients with chronic angle-closure glaucoma combined with cataract. J Korean Ophthalmol Soc. 1996. 37:1045–1053.
19. Nishida T, Saika S. Krachmer JH, Mannis MJ, Holland EJ, editors. Basic science: Cornea, sclera, ocular adnexa anatomy, physiology and pathophysiologic responses. Cornea. 2011. v. 1. Cincinnati: Elsevier;chap. 1.
20. Park WT, Kim KS. The changes of ultrastructure and function of the corneal endothelial cell caused by benzalkonium chloride. J Korean Ophthalmol Soc. 1999. 40:2423–2430.
21. Meyer KT, Pettit TH, Straatsma BR. Corneal endothelial damage with neodymium:YAG laser. Ophthalmology. 1984. 91:1022–1028.
22. Kaji Y, Oshika T, Usui T, Sakakibara J. Effect of shear stress on attachment of corneal endothelial cells in association with corneal endothelial cell loss after laser iridotomy. Cornea. 2005. 24:S55–S58.
23. Hahn YH, Park SK, Hur B. Toxicity of benzalkonium chloride on corneal endothelium of rabbits. J Korean Ophthalmol Soc. 1995. 36:1155–1161.
24. Kerr Muir MG, Sherrard ES. Damage to the corneal endothelium during Nd/YAG photodisruption. Br J Ophthalmol. 1985. 69:77–85.
25. Olsen T. The endothelial cell damage in acute glaucoma. On the corneal thickness response to intraocular pressure. Acta Ophthalmol (Copenh). 1980. 58:257–266.
26. Traverso C, Cohen EJ, Groden LR, et al. Central corneal endothelial cell density after argon laser trabeculoplasty. Arch Ophthalmol. 1984. 102:1322–1324.
27. Dragon DM, Robin AL, Pollack IP, et al. Neodymium:YAG laser iridotomy in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1985. 26:789–796.
28. Bigar F, Witmer R. Corneal endothelial changes in primary acute angle-closure glaucoma. Ophthalmology. 1982. 89:596–599.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download