Abstract
Purpose
To investigate the choroidal thickness changes with the Enhanced Depth Imaging (EDI) technique according to refractive errors and axial length in Korean myopia patients.
Methods
A total of 90 eyes from 90 patients with myopia (between the ages of 19 and 39 years) underwent spectral-domain optical coherence tomography (SD-OCT)-EDI evaluations. Spherical equivalent was measured by cycloplegic refraction and axial length was obtained by IOL master®. The subfoveal choroidal thickness was analyzed according to age, sex, axial length and spherical equivalent by linear correlations.
Results
The average age of all subjects was 25.44 years, mean spherical equivalent was -5.06 diopter (D), mean axial length was 25.70 mm, and mean choroidal thickness was 281.47 µm. In a multiple regression model, the patients' age had no statistical effect on subfoveal choroidal thickness. Subfoveal choroidal thickness decreased by 13.58 µm per -1D increase in refractive errors and by 33.99 µm per 1 mm increase in axial length when adjusted for sex and age. In addition, subfoveal choroidal thickness was 59.82 µm thicker in men than in women when adjusted for axial length and spherical equivalent (p < 0.001).
Figures and Tables
Figure 1
Cross-sectional image of the choroid using enhanced depth imaging optical coherence tomography (OCT). Subfoveal choroidal thickness was measured vertically from the outer border of retinal pigment epithelium (RPE) to the inner border of the sclera (chorioscleral interface).
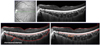
Figure 2
(Top) Subfoveal choroidal thickness shows 305 µm in 25 years old female with axial length (A.L) 24.09 mm and spherical equivalent (S.E) -1.88 D. (Middle) Subfoveal choroidal thickness shows 250 µm in 19 years old female with A.L 24.78 mm and S.E -3.00 D. (Bottom) Subfoveal choroidal thickness shows 140 µm in 35 years old female with A.L 25.65 mm and S.E -7.75 D. As spherical equivalent and axial length of patients increases, subfoveal choroidal thickness has a tendency to decrease.
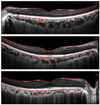
Figure 3
(A) Scatterplot of spherical equivalent and subfoveal choroidal thickness of all subjects shows a significant positive correlation (p < 0.001 : y = 13.58x +347.60; R2 = 0.226). (B) Scatterplot of axial length and subfoveal choroidal thickness of all subjects shows a significant negative correlation (p < 0.001 : y = -33.99x + 897.01; R2 = 0.291).

References
1. Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005. 25:381–391.
2. Klein RM, Curtin BJ. Lacquer crack lesions in pathologic myopia. Am J Ophthalmol. 1975. 79:386–392.
3. Hotchkiss ML, Fine SL. Pathologic myopia and choroidal neovascularization. Am J Ophthalmol. 1981. 91:177–183.
4. Harris A, Bingaman DP, Ciulla TA, Martin BJ. Ryan SJ, Ogden TE, Hinton DR, editors. Retina and choroidal blood flow in health and disease. Retina. 2006. 4th ed. Philadelphia: Elsevier;83–102.
5. Chui TY, Yap MK, Chan HH, Thibos LN. Retinal stretching limits peripheral visual acuity in myopia. Vision Res. 2005. 45:593–605.
6. Atchison DA, Schmid KL, Pritchard N. Neural and optical limits to visual performance in myopia. Vision Res. 2006. 46:3707–3722.
7. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008. 146:496–500.
8. Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004. 137:496–503.
9. Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008. 19:208–212.
10. Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996. 16:203–213.
11. Duke-Elder S, Abrams D. Pathological myopia. Duke-Elder's System of Ophthalmology, Refractive Optics and Refraction. 1970. v. 5. London: Henry Kimpton;308.
12. Kim KH, Kim DG. The relationship among refractive power, axial length and choroidal thickness measured by SD-OCT in Myopia. J Korean Ophthalmol Soc. 2012. 53:626–631.
13. Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010. 51:2173–2176.
14. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009. 147:811–815.
15. Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009. 148:445–450.
16. Agawa T, Miura M, Ikuno Y, et al. Choroidal thickness measurement in healthy Japanese subjects by three-dimensional high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2011. 249:1485–1492.
17. Li XQ, Larsen M, Munch IC. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest Ophthalmol Vis Sci. 2011. 52:8438–8441.
18. Guyer DR, Schachat AP, Green WR. The choroid: structural considerations. Retina. 2006. 4th ed. Philadelphia: Elsevier;33–42.
19. Ikuno Y, Maruko I, Yasuno Y, et al. Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2011. 52:5536–5540.
20. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011. 31:1904–1911.
21. Ramrattan RS, van der Schaft TL, Mooy CM, et al. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994. 35:2857–2864.
22. Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995. 36:579–585.
23. Lütjen-Drecoll E. Choroidal innervation in primate eyes. Exp Eye Res. 2006. 82:357–361.
24. Reitsamer HA, Zawinka C, Branka M. Dopaminergic vasodilation in the choroidal circulation by d1/d5 receptor activation. Invest Ophthalmol Vis Sci. 2004. 45:900–905.
25. Chou PI, Lu DW, Chen JT. Adrenergic supersensitivity of rabbit choroidal blood vessels after sympathetic denervation. Curr Eye Res. 2001. 23:352–356.
26. Shimura M, Uchida S, Suzuki A, et al. Reflex choroidal blood flow responses of the eyeball following somatic sensory stimulation in rats. Auton Neurosci. 2002. 97:35–41.
27. Delgado E, Marques-Neves C, Rocha I, et al. Intrinsic vasomotricity and adrenergic effects in a model of isolated rabbit eye. Acta Ophthalmol. 2009. 87:443–449.
28. Brown JS, Flitcroft DI, Ying GS, et al. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009. 50:5–12.
29. Archer D, Krill AE, Newell FW. Fluorescein studies of normal choroidal circulation. Am J Ophthalmol. 1970. 69:543–554.
30. Tanabe H, Ito Y, Iguchi Y, et al. Correlation between cross-sectional shape of choroidal veins and choroidal thickness. Jpn J Ophthalmol. 2011. 55:614–619.
31. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992. 12:127–133.
32. Okabe S, Matsuo N, Okamoto S, Kataoka H. Electron microscopic studies on retinochoroidal atrophy in the human eye. Acta Med Okayama. 1982. 36:11–21.
33. Hirata A, Negi A. Morphological changes of choriocapillaris in experimentally induced chick myopia. Graefes Arch Clin Exp Ophthalmol. 1998. 236:132–137.
34. Akyol N, Kükner AS, Ozdemir T, Esmerligil S. Choroidal and retinal blood flow changes in degenerative myopia. Can J Ophthalmol. 1996. 31:113–119.
35. To'mey KF, Faris BM, Jalkh AE, Nasr AM. Ocular pulse in high myopia: a study of 40 eyes. Ann Ophthalmol. 1981. 13:569–571.
36. Wakabayashi T, Ikuno Y. Choroidal filling delay in choroidal neovascularisation due to pathological myopia. Br J Ophthalmol. 2010. 94:611–615.
37. Ohno H. Electron microscopic studies of myopic retinochoroidal atrophies 1. Choroidal changes. Folia Ophthalmol Jpn. 1983. 43:1244–1253.
38. Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002. 109:704–711.
39. Kitzmann AS, Pulido JS, Diehl NN, et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008. 115:169–173.
40. Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994. 35:4344–4347.
41. Tsai MY, Lin LL, Lee V, et al. Estimation of heritability in myopic twin studies. Jpn J Ophthalmol. 2009. 53:615–622.
42. Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983. 101:405–407.
43. Smith W, Mitchell P, Wang JJ. Gender, oestrogen, hormone replacement and age-related macular degeneration: results from the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1997. 25:Suppl 1. S13–S15.
44. Cho JH, Bae SH, Han JR, et al. Comparison of choroidal thickness in eyes with central serous chorioretinopathy, asymptomatic fellow eyes and normal eyes. J Korean Ophthalmol Soc. 2012. 53:87–93.
45. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010. 117:1792–1799.
46. Pryds A, Larsen M. Choroidal thickness following extrafoveal photodynamic treatment with verteporfin in patients with central serous chorioretinopathy. Acta Ophthalmol. 2011. 05. 17. doi: 10.1111/j.1755-3768.2011.02157.x. [Epub ahead of print].
47. Dirani M, Chamberlain M, Shekar SN, et al. Heritability of refractive error and ocular biometrics: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2006. 47:4756–4761.
48. Munaut C, Lambert V, Noël A, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol. 2001. 85:877–882.
49. Kavroulaki D, Gugleta K, Kochkorov A, et al. Influence of gender and menopausal status on peripheral and choroidal circulation. Acta Ophthalmol. 2010. 88:850–853.
50. Centofanti M, Bonini S, Manni G, et al. Do sex and hormonal status influence choroidal circulation? Br J Ophthalmol. 2000. 84:786–787.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download