Abstract
Purpose
To report a case of keratoconjunctival chemical injury caused by exposure to EMLA® 5% cream.
Case summary
A 51-year-old woman presented with ocular pain and decreased visual acuity in her left eye after an autologous fat injection for forehead lifting. At her initial visit, her best corrected visual acuity was 20/40 in the left eye. Slit-lamp examination showed a diffuse corneal epithelial defect and conjunctival injection. Based on history of inadvertent seepage of EMLA® 5% cream into the left eye and clinical findings consistent with chemical injury, the patient was treated with antibiotics, steroids, and artificial tears. Two weeks after treatment, several corneal erosions remained, and best corrected visual acuity improved to 20/20. After two months, the corneal and conjunctival epithelia were healed.
Figures and Tables
Figure 1
Slit-lamp photographs of initial presentation showing diffuse conjunctival injection, mild corneal edema (A) and corneal epithelial defect with distinct margin (B). Slit-lamp photographs of conjunctiva showing epithelial defect at 7 o'clock (C) and at 3 o'clock (D) without limbal ischemia.
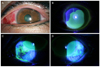
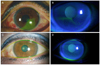
Figure 2
At three days after treatment, the left eye presented conjunctival injection and decreased corneal epithelial defect (A). After two weeks, conjunctival injection was nearly disappeared, and there was no epithelial defect in the cornea and conjunctiva. Some corneal erosions remained alone (B). At two months, conjunctival injection and corneal erosions were disappeared (C, D).
References
1. Gupta AK, Koren G, Shear NH. A double-blind, randomized, placebo-controlled trial of eutectic lidocaine/prilocaine cream 5% (EMLA) for analgesia prior to cryotherapy of warts in children and adults. Pediatr Dermatol. 1998. 15:129–133.
2. Goldman MP, Fitzpatrick RE. Cutaneous Laser Surgery : the art and science of selective photothermolysis. 1994. St. Louis: Mosby Year Book;270–272.
3. Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995. 95:776–778.
4. Pfister RR, Koski J. Alkali burns of the eye: pathophysiology and treatment. South Med J. 1982. 75:417–422.
5. Pfister RR. Chemical injuries of the eye. Ophthalmology. 1983. 90:1246–1253.
6. Kim SS, Yoo JM. A clinical study of industrial ocular injuries. J Korean Ophthalmol Soc. 1988. 29:393–403.
7. Friedenwald JS, Hughes WF Jr, Herrmann H. Acid burns of the eye. Arch Ophthal. 1946. 35:98–108.
8. Gnädinger MC, Itoi M, Slansky HH, Dohlman CH. The role of collagenase in the alkali-burned cornea. Am J Ophthalmol. 1969. 68:478–483.
9. Astra Pharmaceuticals Ltd. EMLA Cream 5%, ABPT Data Sheet Compendium. 1993. 4. London: Datapharm Publications;107.
10. EMLA® Anaesthesia for Superficial Skin Surgery. 1993. Oxford: Oxford Clinical Communications;1–48.
11. Juhlin L, Evers H. EMLA: a new topical anesthetic. Adv Dermatol. 1990. 5:75–91. discussion 92.
12. Van den Hove J, Decroix J, Tennstedt D, Lachapelle JM. Allergic contact dermatitis from prilocaine, one of the local anaesthetics in EMLA cream. Contact Dermatitis. 1994. 30:239.
13. García F, Iparraguirre A, Blanco J, et al. Contact dermatitis from prilocaine with cross-sensitivity to pramocaine and bupivacaine. Contact Dermatitis. 2007. 56:120–121.
14. Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009. 7:237–238.
15. Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from Emla cream. Contact Dermatitis. 2004. 51:284–287.
16. Dong H, Kerl H, Cerroni L. EMLA cream-induced irritant contact dermatitis. J Cutan Pathol. 2002. 29:190–192.
17. Kluger N, Raison-Peyron N, Michot C, et al. Acute bullous irritant contact dermatitis caused by EMLA® cream. Contact Dermatitis. 2011. 65:181–183.
18. Palao R, Monge I, Ruiz M, Barret JP. Chemical burns: pathophysiology and treatment. Burns. 2010. 36:295–304.
19. Brahma AK, Inkster C. Alkaline chemical ocular injury from Emla cream. Eye (Lond). 1995. 9(Pt 5):658–659.
20. Gotsis SS, Volonaki OM, Theodossiadis GP. Percutaneous anaesthesia with a lignocaine-prilocaine cream (Emla) for eyelid skin surgery. Br J Ophthalmol. 1994. 78:209–210.
21. Chevaleraud E, Leroy L, Lebuisson DA. [EMLA cream: prudent use and warnings]. Ann Fr Anesth Reanim. 1995. 14:459.
22. Eaglstein NF. Chemical injury to the eye from EMLA cream during erbium laser resurfacing. Dermatol Surg. 1999. 25:590–591.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download