Abstract
Purpose
To investigate clinical manifestations and prognostic factors of autoimmune-related peripheral corneal ulcers.
Methods
Nineteen eyes in 18 patients who were diagnosed with autoimmune-related peripheral corneal ulcer from November 1999 to August 2010 were enrolled in the present study. Clinical manifestations and treatment results were investigated retrospectively.
Results
The average age at presentation was 64.6 years with female (66.7%) and unilateral (94.4%) dominance. The main etiologies were Mooren's ulcer (53.6%) and rheumatoid arthritis (26.3%). The ulcer depth was greater than 75% of the corneal thickness in more than half of the cases (57.9%) and the mean extent of the ulcer was 69.5°. There were no significant improvements in visual acuity after treatment (p = 0.789) and no significant differences in treatment outcomes among etiologies or treatment modalities. The patients who underwent ulcer recurrence (p = 0.048) or treatment failure (p = 0.005) had poorer final visual acuity than those patients who did not. The ulcer depth correlated with treatment failure (p = 0.037). The final visual acuity showed positive correlations with visual acuity at presentation (p = 0.031) and negative correlations with the number of recurrences (p = 0.042).
Go to : 
References
2. Allansmith MR, McClellan BH. Immunoglobulins in the human cornea. Am J Ophthalmol. 1975; 80:123–32.

3. Lewallen S, Courtright P. Problems with current concepts of the epidemiology of Mooren's corneal ulcer. Ann Ophthalmol. 1990; 22:52–5.
5. Messmer EM, Foster CS. Vasculitic peripheral ulcerative keratitis. Surv Ophthalmol. 1999; 43:379–96.

6. Ladas JG, Mondino BJ. Systemic disorders associated with peripheral corneal ulceration. Curr Opin Ophthalmol. 2000; 11:468–71.

7. Malik R, Culinane AB, Tole DM, Cook SD. Rheumatoid keratol-ysis: a series of 40 eyes. Eur J Ophthalmol. 2006; 16:791–7.

8. Chen KH, Hsu WM, Liang CK. Relapsing Mooren's ulcer after amniotic membrane transplantation combined with conjunctival autografting. Ophthalmology. 2004; 111:792–5.
10. Chen J, Xie H, Wang Z, et al. Mooren's ulcer in China: a study of clinical characteristics and treatment. Br J Ophthalmol. 2000; 84:1244–9.

11. Woo SJ, Wee WR, Lee JH. The clinical manifestations of Mooren's ulcer. J Korean Ophthalmol Soc. 2002; 43:1388–96.
12. Kim DG, Kim BJ. A peripheral corneal ulcer in rheumatoid arthritis. J Korean Ophthalmol Soc. 1987; 28:1083–7.
13. Kervick GN, Pflugfelder SC, Haimovici R, et al. Paracentral rheumatoid corneal ulceration. Clinical features and cyclosporine therapy. Ophthalmology. 1992; 99:80–8.
14. Bernauer W, Ficker LA, Watson PG, Dart JK. The management of corneal perforations associated with rheumatoid arthritis. An analysis of 32 eyes. Ophthalmology. 1995; 102:1325–37.
15. Messmer EM, Foster CS. Destructive corneal and scleral disease associated with rheumatoid arthritis. Medical and surgical management. Cornea. 1995; 14:408–17.
16. Tauber J, Sainz de la Maza M, Hoang-Xuan T, Foster CS. An analysis of therapeutic decision making regarding immunosuppressive chemotherapy for peripheral ulcerative keratitis. Cornea. 1990; 9:66–73.

17. Gottsch JD, Liu SH, Minkovitz JB, et al. Autoimmunity to a cornea-associated stromal antigen in patients with Mooren's ulcer. Invest Ophthalmol Vis Sc. 1995; 36:1541–7.
18. Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000; 19:625–43.
19. Feder RS, Krachmer JH. Conjunctival resection for the treatment of the rheumatoid corneal ulceration. Ophthalmology. 1984; 91:111–5.

20. Fogle JA, Kenyon KR, Foster CS. Tissue adhesive arrests stromal melting in the human cornea. Am J Ophthalmol. 1980; 89:795–802.

21. Bessant DA, Dart JK. Lamellar keratoplasty in the management of inflammatory corneal ulceration and perforation. Eye. 1994; 8:22–8.

22. Raizman MB, Sainz de la Maza M, Foster CS. Tectonic keratoplastyaccommodative for peripheral ulcerative keratitis. Cornea. 1991; 10:312–6.
Go to : 
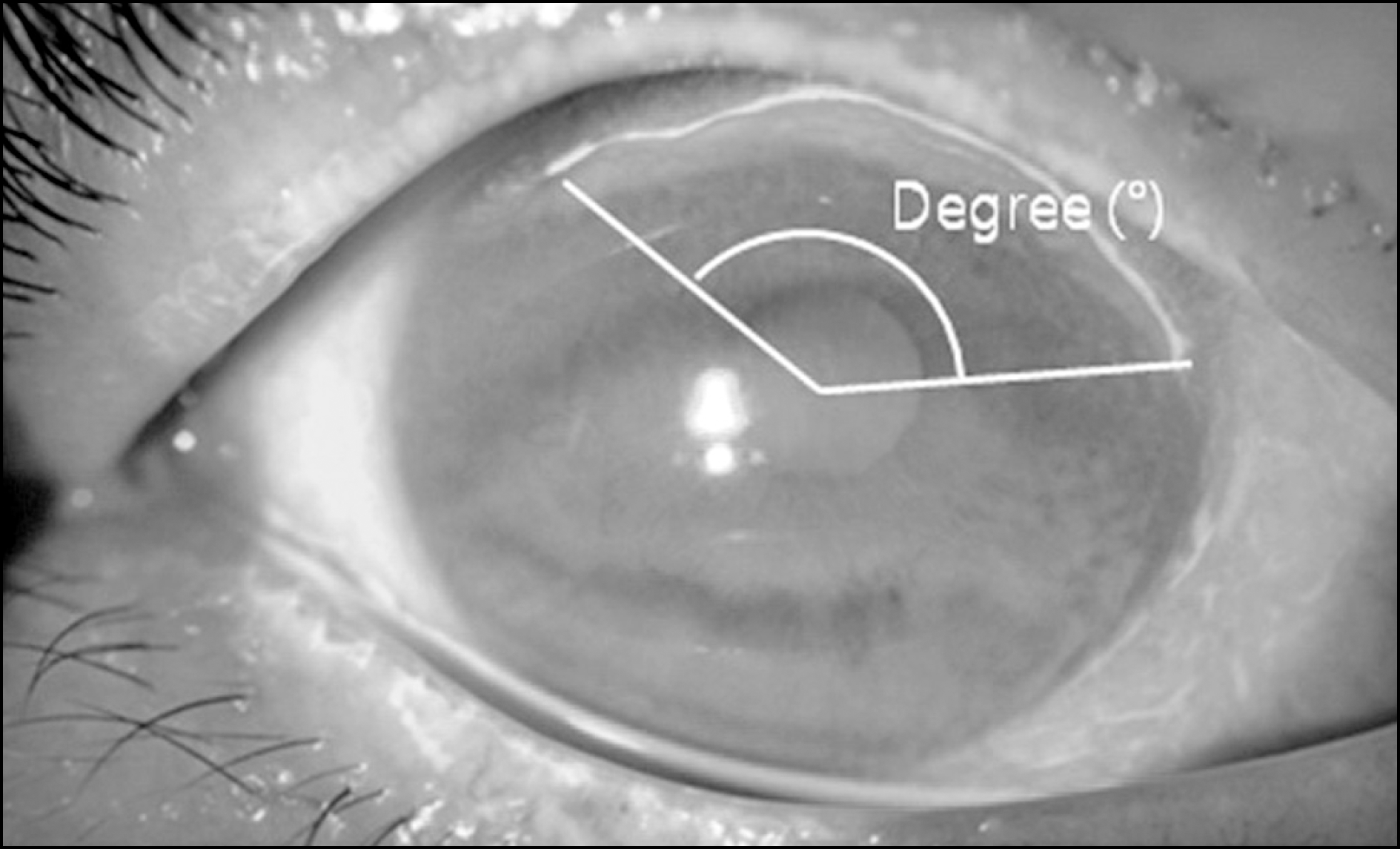 | Figure 1.Corneal ulcer extent. The angle between the two margins of the corneal ulcer (degree). |
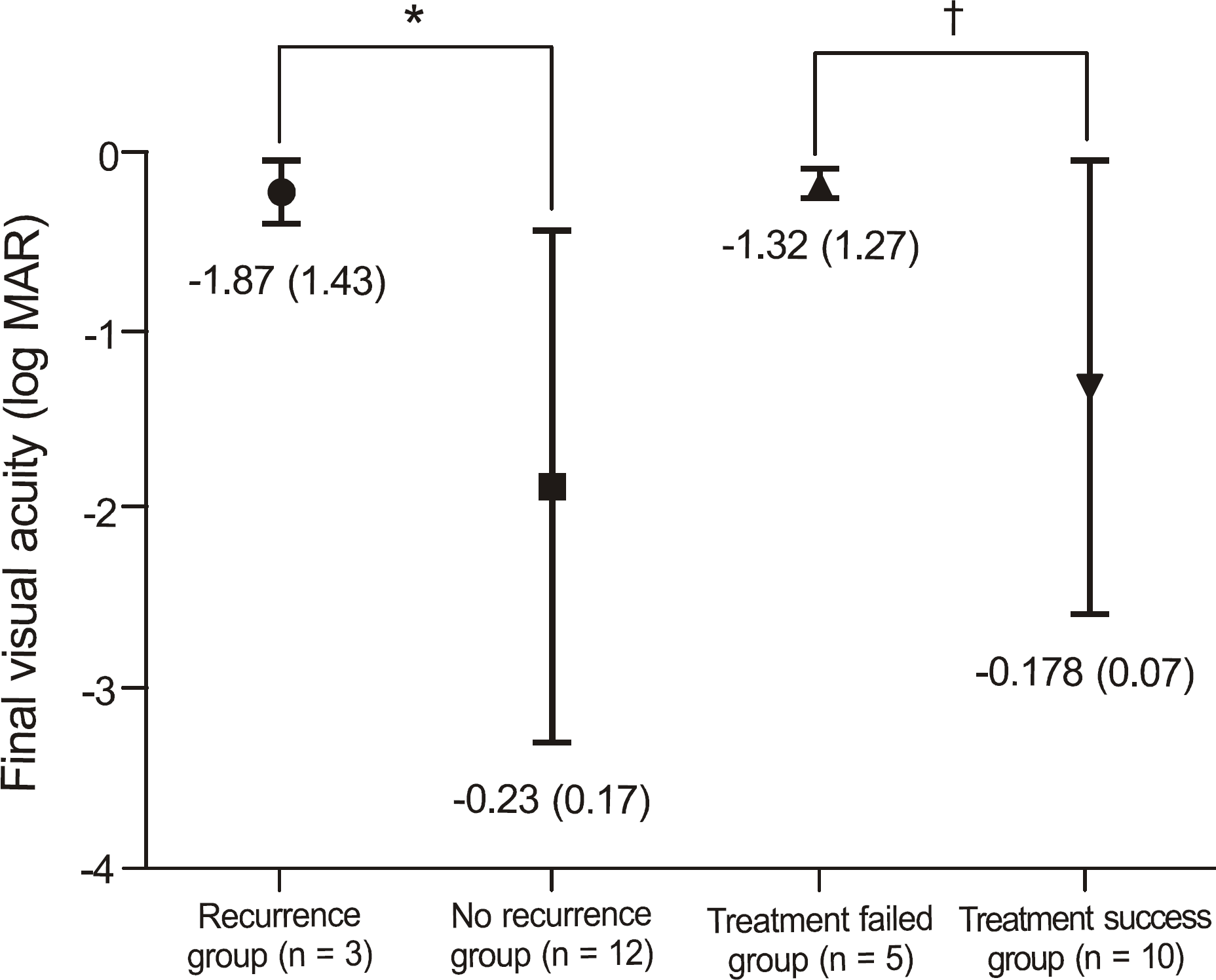 | Figure 2.Comparison of final acuity for recurrence or treatment failure. Statistics were analyzed by Mann-Whitney U-test. * p = 0.048; † p = 0.005. |
Table 1.
Clinical features of autoimmune related peripheral corneal ulcer
Table 2.
Range of the treatment used
| Variable | Operation | No. (%) of patients |
|---|---|---|
| Surgical treatment (n = 22) | Amniotic membrane transplantation | 14 (48.3%) |
| | Glue application | 6 (20.7%) |
| | Corneal limbal transplantation | 5 (17.2%) |
| | Scleral patch graft | 2 (6.9%) |
| | Conjunctival recession | 2 (6.9%) |
| | Total | 29* |
| Topical immune-suppressive treatment (n = 20) | Steroid | 20 (60.6%) |
| | Cyclosporin A | 10 (30.3%) |
| | Interferon-α | 2 (6.1%) |
| | Taclolimus | 1 (3.0%) |
| | Total | 33* |
| Systemic immune-suppressive treatment (n = 19) | Steroid | 19 (59.4%) |
| | Cyclosporin A | 7 (18.6%) |
| | Mycophenolate mofetil | 4 (12.5%) |
| | Cyclophosphamide | 1 (3.9%) |
| | Infliximab | 1 (3.9%) |
| | Total | 32* |
Table 3.
The comparison of treatment outcomes between treatment modalities
| Variable | Surgical and medical treatment (n = 7) | Medical treatment only (n = 7) | p-value | Total (n = 15*) |
|---|---|---|---|---|
| Visual acuity at presentation (log MAR) | −0.63 (0.26) | −0.37 (0.59) | 0.038 | −0.50 (0.44) |
| Final visual acuity (log MAR) | −0.98 (−1.18) | −0.18 (0.08) | 0.073 | −0.56 (0.88) |
| p-value | 0.335 | 0.371 | | 0.789 |
| Duration of treatment (mon) | 2.02 (1.50) | 0.88 (0.58) | 0.165 | 1.42 (1.20) |
| Number of recurrence | 0.43 (0.79) | 0.00 (0.00) | 0.383 | 0.27 (0.59) |
| Number of treatment failure | 1.43 (1.27) | 0.00 (0.00) | 0.026 | 0.67 (1.11) |
| Visually improved after treatment:not improved | 4 (57.1%):3 (42.9%) | 5 (71.4%):2 (28.6%) | 0.296 | 6 (42.9%):8 (57.1%) |
Table 4.
Comparison of treatment outcomes between etiologies
Table 5.
Comparison of treatment outcome for clinical characteristics
| |
Site of ulcer |
Onset of ulcer |
New vessel ingrowth* |
Sign of inflammation* |
Ulcer depth |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal (n = 5) | Superior (n = 5) | Inferior (n = 5) | p | Acute (n = 5) | Chronic (n = 10) | p | Mild†(n = 6) | Severe†(n = 8) | p | Mild‡(n = 7) | Severe‡(n = 5) | p | Mild§(n = 7) | Severe§(n = 8) | p | |
| Final visual acuity (log MAR) | −0.29(0.24) | −0.66(1.14) | −0.72(1.10) | 0.641 | −0.70(1.12) | −0.49(0.80) | 0.679 | −1.03 (1.29) | −0.26(1.88) | 1.000 | −0.53 (0.96) | −0.31 (0.24) | 0.639 | −0.18(0.86) | −0.89(1.13) | 0.072 |
| Number of recurrence | 0.00 (0.00) | 0.60(0.89) | 0.20(0.45) | 0.291 | 0.60(0.89) | 0.10(0.32) | 0.371 | 0.50 (0.84) | 0.13(0.35) | 0.491 | 0.43(0.79) | 0.00(0.00) | 0.432 | 0.00(0.00) | 0.50 (0.76) | 0.232 |
| Number of treatment failure | 0.80 (1.30) | 0.40(0.89) | 0.80(1.30) | 0.766 | 0.40 (0.89) | 0.80(1.23) | 0.594 | 0.83(1.33) | 0.63(1.06) | 0.950 | 0.71(1.25) | 0.40(0.55) | 1.000 | 0.00 (0.00) | 1.25(1.28) | 0.040 |
Table 6.
Associations of final visual acuity with clinical characteristics by linear regression analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download