Abstract
Purpose
To develop a new decellularization technique of porcine cornea using freezing-thawing-centrifugation (FTC) and to examine the characteristics of acellular porcine cornea (APC) for xenograft material.
Methods
Two-hundred micrometer thickness porcine corneas were decellularized with DNase/RNase, followed by 3 freezing-thawing-centrifugations (FTC, group 1), lyophilized FTC-APC (group 2), and chemical enzyme treated APC (CE-APC, group 3). Histologic evaluation to examine cells and collagen matrix, comparison of transparency, and cultivation to determine the viability of stromal cells was performed in fresh porcine cornea and 3 experimental groups.
Results
Decellularization occurred successfully in all experimental groups. Decellularization was confirmed by H&E staining and cultivation. Transparency of group 1 was similar to the normal porcine cornea but transparency of group 2 and group 3 was decreased. Collagen fibers of CE-APC (group 3) were not as well arrayed as FTC-APC (group 2).
Go to : 
References
1. Sedlakova K, Filipec M. Effect of suturing technique on corneal xenograft survival. Cornea. 2007; 26:1111–4.

2. Lee JK, Ryu YH, Ahn JI, et al. The effect of lyophilization on graft acceptance in experimental xenotransplantation using porcine cornea. Artif Organs. 2010; 34:37–45.

3. Choi SH, Lee YW, Kim HM, et al. Epidemiologic studies of keratoplasty in Korea. J Korean Ophthalmol Soc. 2006; 47:538–47.
4. Melles GR, Remeijer L, Geerards AJ, Beekhuis WH. A quick surgical technique for deep anterior lamellar keratoplasty using viscodissection. Cornea. 2000; 19:427–32.

5. Lin XC, Hui YN, Wang YS, et al. Lamellar keratoplasty with a graft of lyophilized acellular porcine corneal stroma in the rabbit. Vet Ophthalmol. 2008; 11:61–6.

6. Amano S, Shimomura N, Kaji Y, et al. Antigenicity of porcine cornea as xenograft. Curr Eye Res. 2003; 26:313–8.

7. Xu YG, Xu YS, Huang C, et al. Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate. Mol Vis. 2008; 14:2180–9.
8. Lee HI, Kim MK, Ko JH, et al. The Characteristics of Porcine Cornea as a Xenograft. J Korean Ophthalmol Soc. 2006; 47:2020–9.
9. Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006; 27:3675–83.

10. López-García JS, Rivas Jara L, García-Lozano I, et al. aberrations limbus evolution after alkaline burns. Cornea. 2007; 26:1043–8.
11. Kim MS, Lee SC, Lee SJ, JIN KH. Predictability of Donor Lamellar Graft Thickness and Diameter Using a Microkeratome in Porcine Eyes. J Korean Ophthalmol Soc. 2007; 48:473–7.
12. D'Amico RA, Chaunico R, Castroviejo R. Suppression of the immune response to corneal xenografts. Transplant Proc. 1969; 1:256–8.
14. Insler MS, Lopez JG. Heterologous transplantation versus enhancement of human corneal endothelium. Cornea. 1991; 10:136–48.

15. Ross JR, Howell DN, Sanfilippo FP. Characteristics of corneal xenograft rejection in a discordant species combination. Invest Ophthalmol Vis Sci. 1993; 34:2469–76.
16. Li C, Xu JT, Kong FS, Li JL. Experimental studies on penetrating heterokeratoplasty with human corneal grafts in monkey eyes. Cornea. 1992; 11:66–72.

17. Tseng YL, Kuwaki K, Dor FJ, et al. Alpha1, 3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005; 80:1493–500.
18. Rosenbluth J, Schiff R, Liang WL, et al. Xenotransplantation of transgenic oligodendrocyte-lineage cells into spinal cord-injured adult rats. Exp Neurol. 1997; 147:172–82.

19. Tanaka K, Yamada J, Streilein JW. Xenoreactive CD4+ T cells and acute rejection of orthotopic guinea pig corneas in mice. Invest Ophthalmol Vis Sci. 2000; 41:1827–32.
20. Freytes DO, Badylak SF, Webster TJ, et al. Biaxial strength of mul-tilaminated extracellular matrix scaffolds. Biomaterials. 2004; 25:2353–61.

21. De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002; 168:1789–92.

22. Jackson DW, Grood ES, Cohn BT, et al. The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg Am. 1991; 73:201–13.

23. Gulati AK. Evaluation of acellular and cellular nerve grafts in repair of rat peripheral nerve. J Neurosurg. 1988; 68:117–23.

24. Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008; 372:2023–30.

25. Freytes DO, Badylak SF, Webster TJ, et al. Biaxial strength of mul-tilaminated extracellular matrix scaffolds. Biomaterials. 2004; 25:2353–61.

26. Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004; 10:1046–53.

27. Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation. 1997; 95:479–88.
Go to : 
 | Figure 1.Optical transparency of the porcine cornea. Freezing-thawing-centrifugation-decellurized acellular porcine cornea (FTC-APC) was transparent (B), similar with fresh porcine cornea (A). But Transparency of chemical enzyme-decellularized acellular porcine cornea (CE-APC) decreased about one-third compared to fresh porcine cornea (D). Lyophilized FTC-APC was visually opaque (C). |
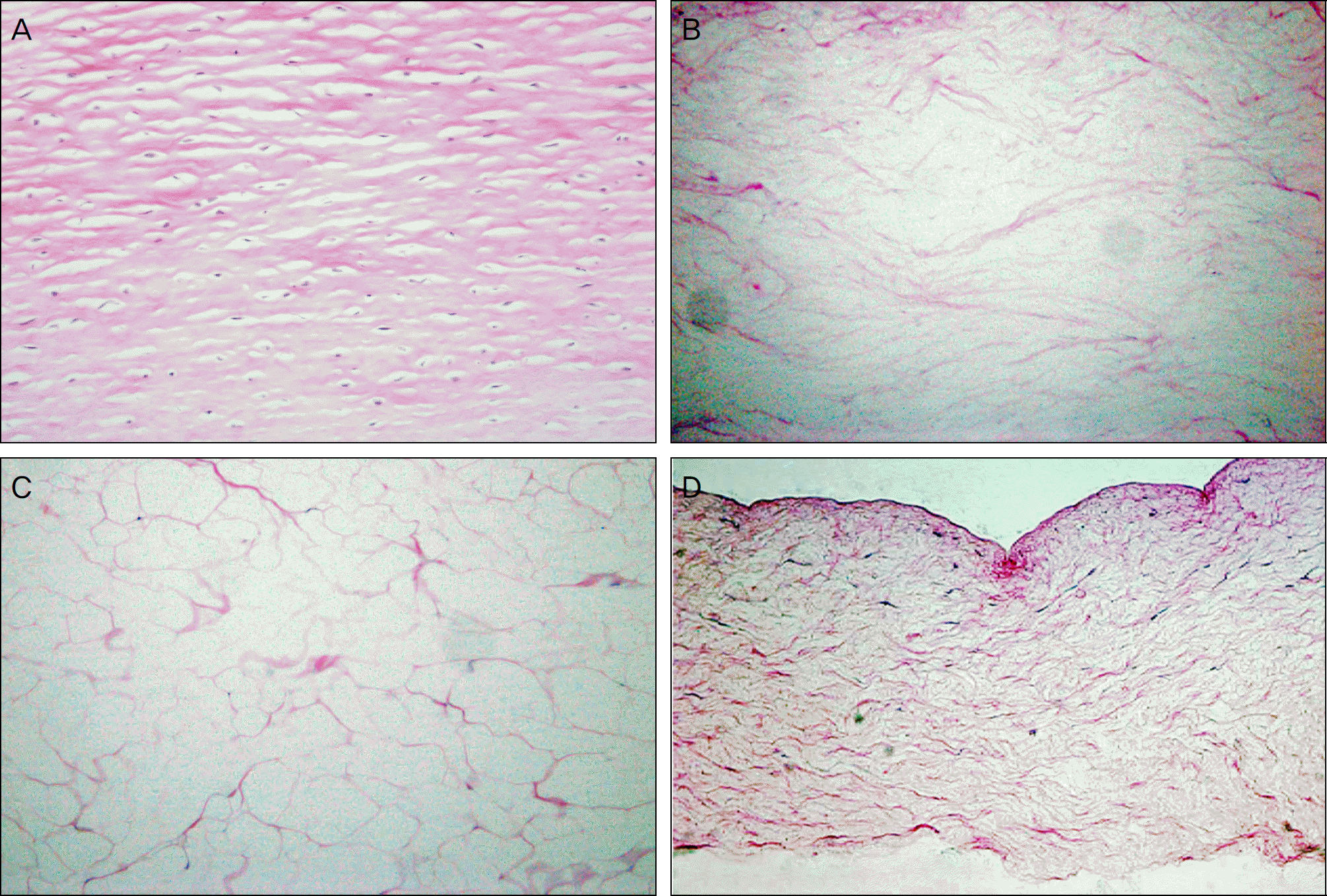 | Figure 2.H&E staining showed that no cells were present in Freezing-thawing-centrifugation-decellurized acellular porcine cornea (FTC-APC) (B), lyophilized FTC-APC (C), and chemical enzyme-decellularized acellular porcine cornea (CE-APC) (D), while many keratocytes were observed in fresh porcine cornea (A). The thickness of CE-APC was thinner than fresh porcine cornea and FTC-APC (A, B, C, D: 200×). |
 | Figure 3.M-T staining showed that collagen fibers were not well arrayed after decellularizing procedure (B: Freezing-thawing-centrifugation-decellurized acellular porcine cornea (FTC-APC), C: lyophilized FTC-APC, D: chemical enzyme-decellular-ized acellular porcine cornea (CE-APC)) than fresh cornea (A). Collagen fibers of CE-APC were most irregular (A, B, C, D: 200×). |
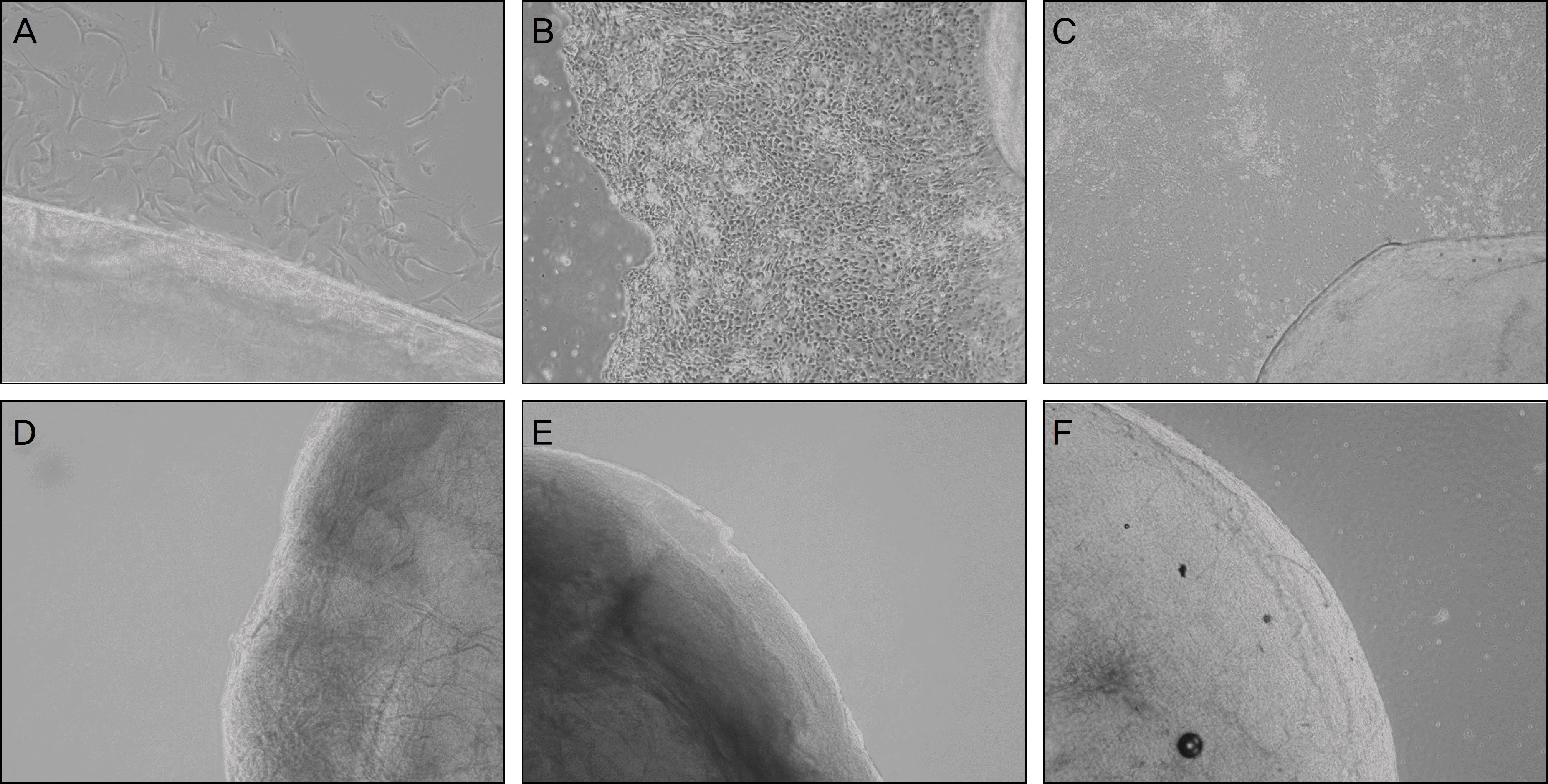 | Figure 4.Keratocytes grew from fresh porcine cornea rapidly (A: Day 1, B: Day 5, C: Day 9). But keratocyte was not grown from Freezing-thawing-centrifugation-decellurized acellular porcine cornea (FTC-APC) (D), lyophilized FTC-APC (E), and chemical en-zyme-decellularized acellular porcine cornea (F) until culture day 9. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download