Abstract
Purpose
To investigate the biologic effects of topical calf serum on corneal epithelial cells in vitro.
Methods
The effects of calf serum on the corneal epithelial cells were evaluated using the MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay, and the concentration of IL-1α, TGF-β1 and MMP-9 in the cells was measured. Cell damage was determined using lactate dehydrogenase (LDH), and cellular morphologies were examined by transmission electromicroscopy.
Results
Metabolic activity of the corneal epithelial cells decreased at higher concentrations and longer exposure durations. IL-1α, TGF-β1 and MMP-9 titers were lower in calf serum-treated cells than in the control. LDH and cellular damage to the corneal epithelial cells, such as chromatin margination and cytoplasmic organelle swelling, were prominent in cells treated with 30% calf serum.
Conclusions
Cellular metabolic activity was higher and cellular toxicity was lower in cells treated with 10% calf serum compared to those treated with the 20% and 30% concentrations. Furthermore, inflammatory cytokines were sufficiently inhibited in cells treated with the 10% solution. These results indicate that 10% calf serum could be used clinically.
Go to : 
References
1. Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004; 88:647–52.

2. Ohashi Y, Motokura M, Kinoshita Y, et al. Presence of epidermal growth factor in human tears. Invest Ophthalmol Vis Sci. 1989; 30:1879–82.
3. Ubels JL, Foley KM, Rismondo V. Retinol secretion by the lacrimal gland. Invest Ophthalmol Vis Sci. 1986; 27:1261–8.
4. Liu L, Hartwig D, Harloff S, et al. An optimised protocol for the production of autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol. 2005; 243:706–14.

5. Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001; 85:1188–97.

6. Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004; 111:1115–20.
7. Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984; 27:459–61.

8. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren's syndrome. Br J Ophthalmol. 1999; 83:390–5.

9. Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999; 106:1984–9.

10. Lee GA, Chen SX. Autologous serum in the management of recalcitrant dry eye syndrome. Clin Experiment Ophthalmol. 2008; 36:119–22.

11. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

12. Wilson SE. Lacrimal gland epidermal growth factor production and the ocular surface. Am J Ophthalmol. 1991; 111:763–5.

13. Collins MK, Perkins GR, Rodriguez-Tarduchy G, et al. Growth factors as survival factors: regulation of apoptosis. Bioessays. 1994; 16:133–8.

14. Fredj-Reygrobellet D, Plouet J, Delayre T, et al. Effects of aFGF and bFGF on wound healing in rabbit corneas. Curr Eye Res. 1987; 6:1205–9.

15. Phan TM, Foster CS, Boruchoff SA, et al. Topical fibronectin in the treatment of persistent corneal epithelial defects and trophic ulcers. Am J Ophthalmol. 1987; 104:494–501.

16. Yoo JW, Chung JH, Lee HR. Effects of topically applied autologous serum on experimental corneal epithelial healing following alkali wounds. J Korean Ophthalmol Soc. 1998; 39:2003–12.
17. Han MS, Lee JH, Lee SJ. Therapeutic effect of topical autologous serum in recurrent punctate corneal erosion. J Korean Ophthalmol Soc. 2004; 45:1639–44.
18. Yoon KC, Im SK, Park YG, Choi J. Tear film and ocular surface changes after umbilical cord serum therapy in dry eye syndrome. J Korean Ophthalmol Soc. 2005; 46:237–42.
19. Eberle J, Habermann J, Gürtler LG. HIV-1 infection transmitted by serum droplets into the eye: a case report. AIDS. 2000; 14:206–7.

20. Egger SF, Huber-Spitzy V, Alzner E, et al. Corneal wound healing after superficial foreign body injury: vitamin A and dexpanthenol versus a calf blood extract. A randomized double-blind study. Ophthalmologica. 1999; 213:246–9.
21. Spessotto P, Dri P, Baschong W, et al. Effect of a protein-free dial-ysate from calf blood on human monocyte differentiation in vitro. Arzneimittelforschung. 1993; 43:747–51.
22. Kojima T, Higuchi A, Goto E, et al. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008; 27:S25–30.

23. Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999; 19:201–11.

24. Sobrin L, Liu Z, Monroy DC, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000; 41:1703–9.
Go to : 
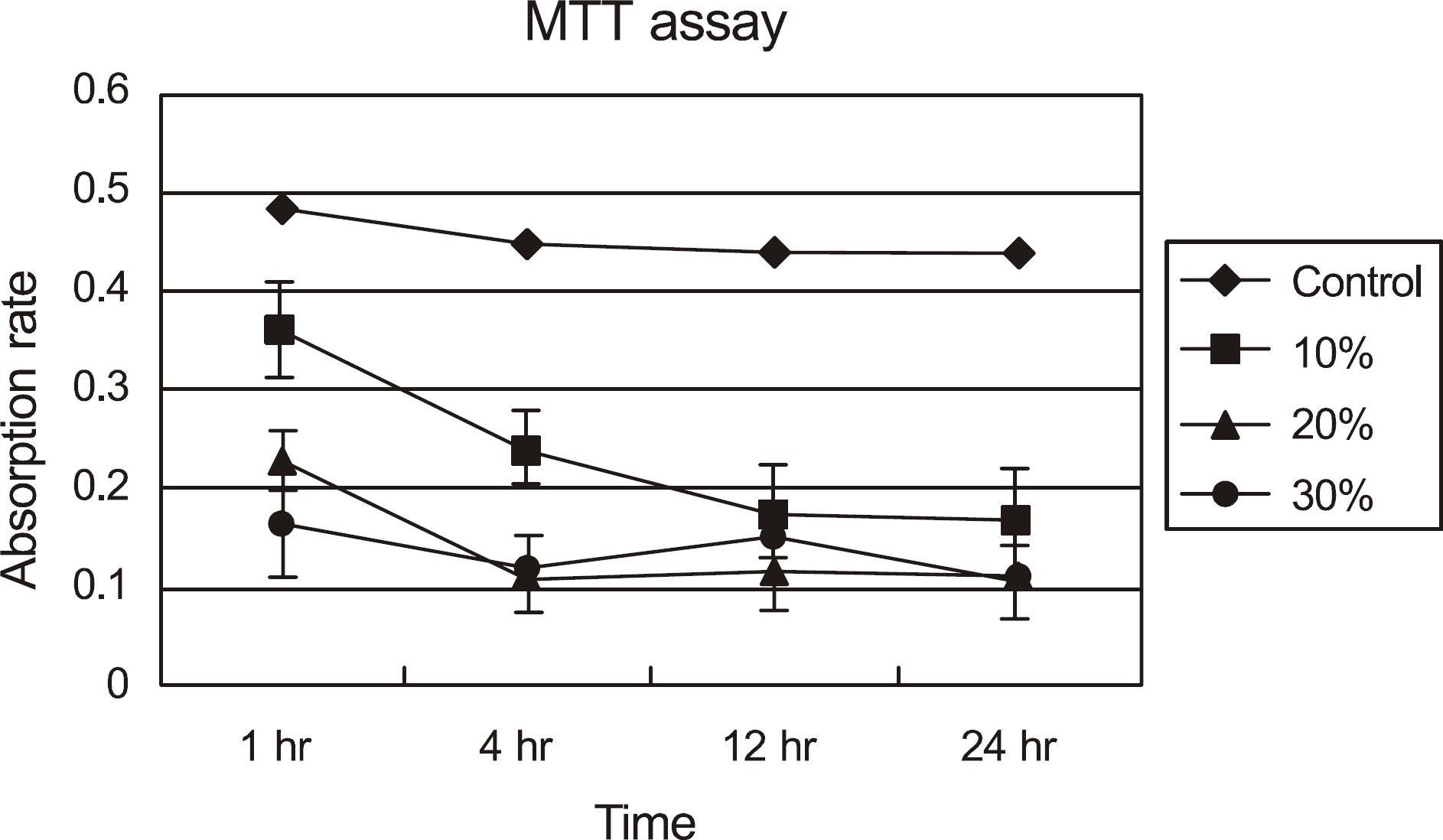 | Figure 1.The absorption rate of the water-insoluble formazan dye in corneal epithelial cell exposed to calf serum by a scanning spectrometer (ELISA reader). Metabolic activity of corneal epithelial cells decreased, at the higher concentration and longer exposure duration. |
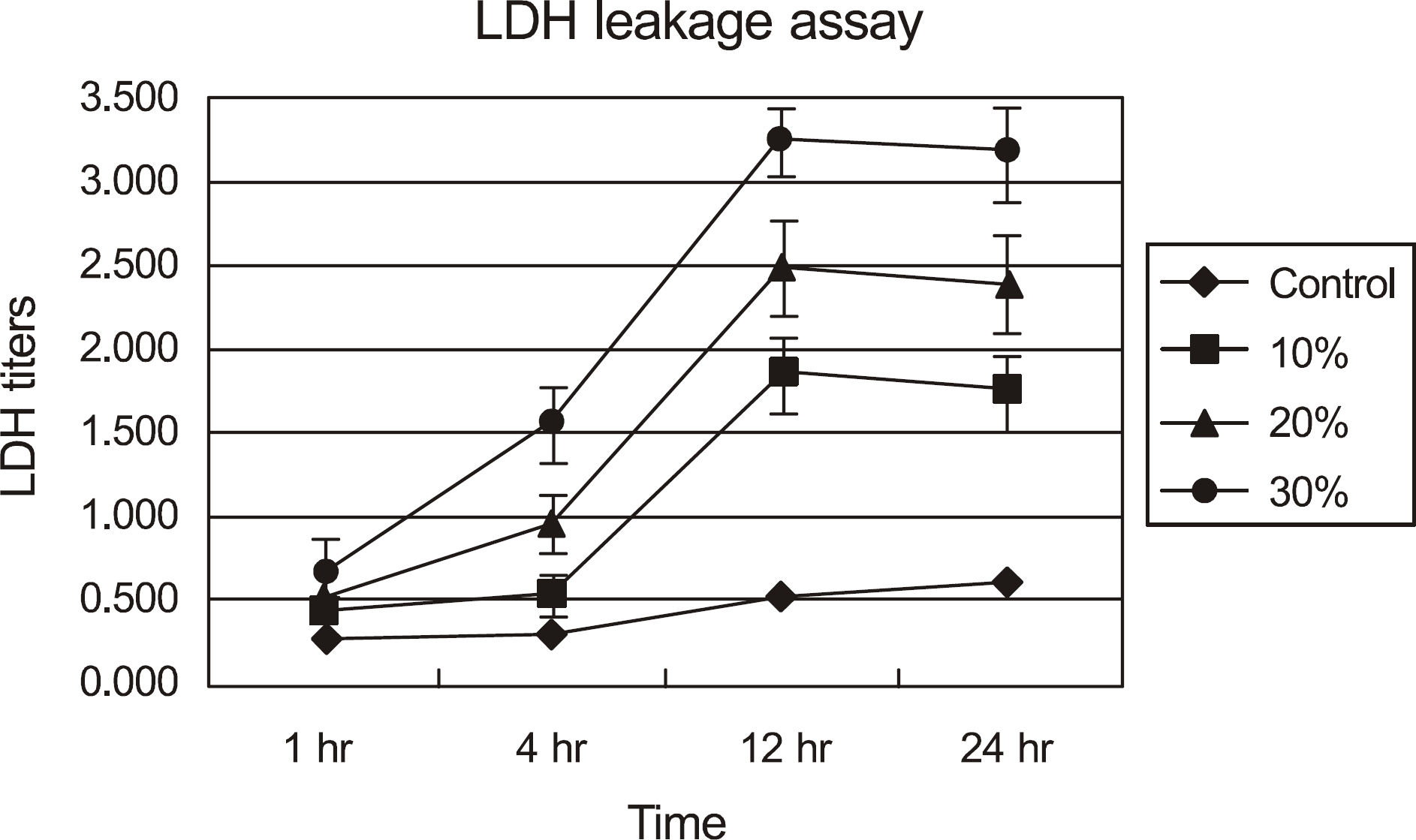 | Figure 2.LDH titers of cultured corneal epithelial cells exposed to calf serum. The LDH titers have showed that by dose-and time-dependent response relationship. LDH = lactate dehydrogenase. |
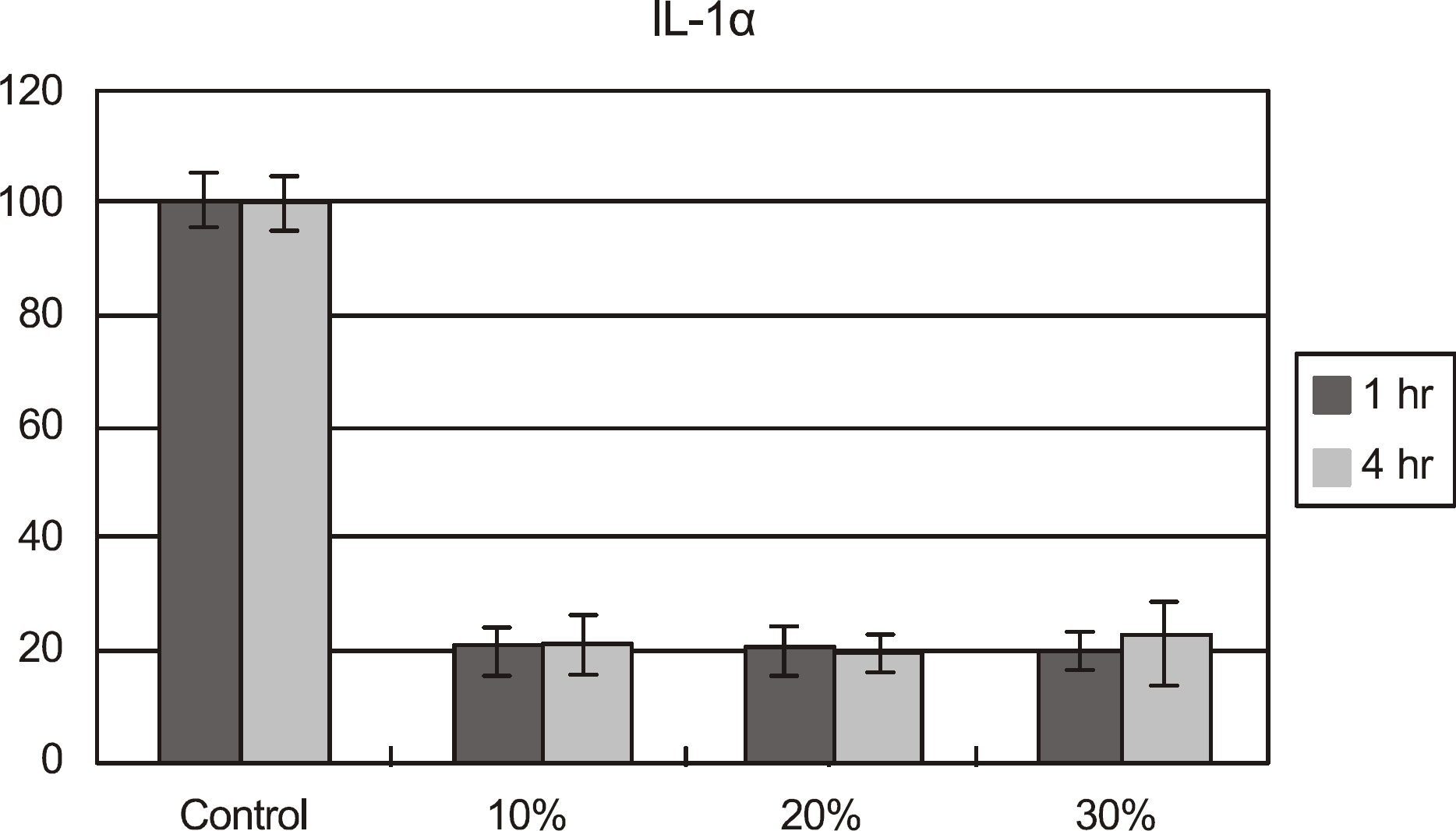 | Figure 3.IL-1α concentration of cultured corneal epithelial cells after exposed to calf serum. The concentration of IL-1α decreased significantly compared to that in the control after exposed to all the concentrations of calf serum for up to 4 hours. |
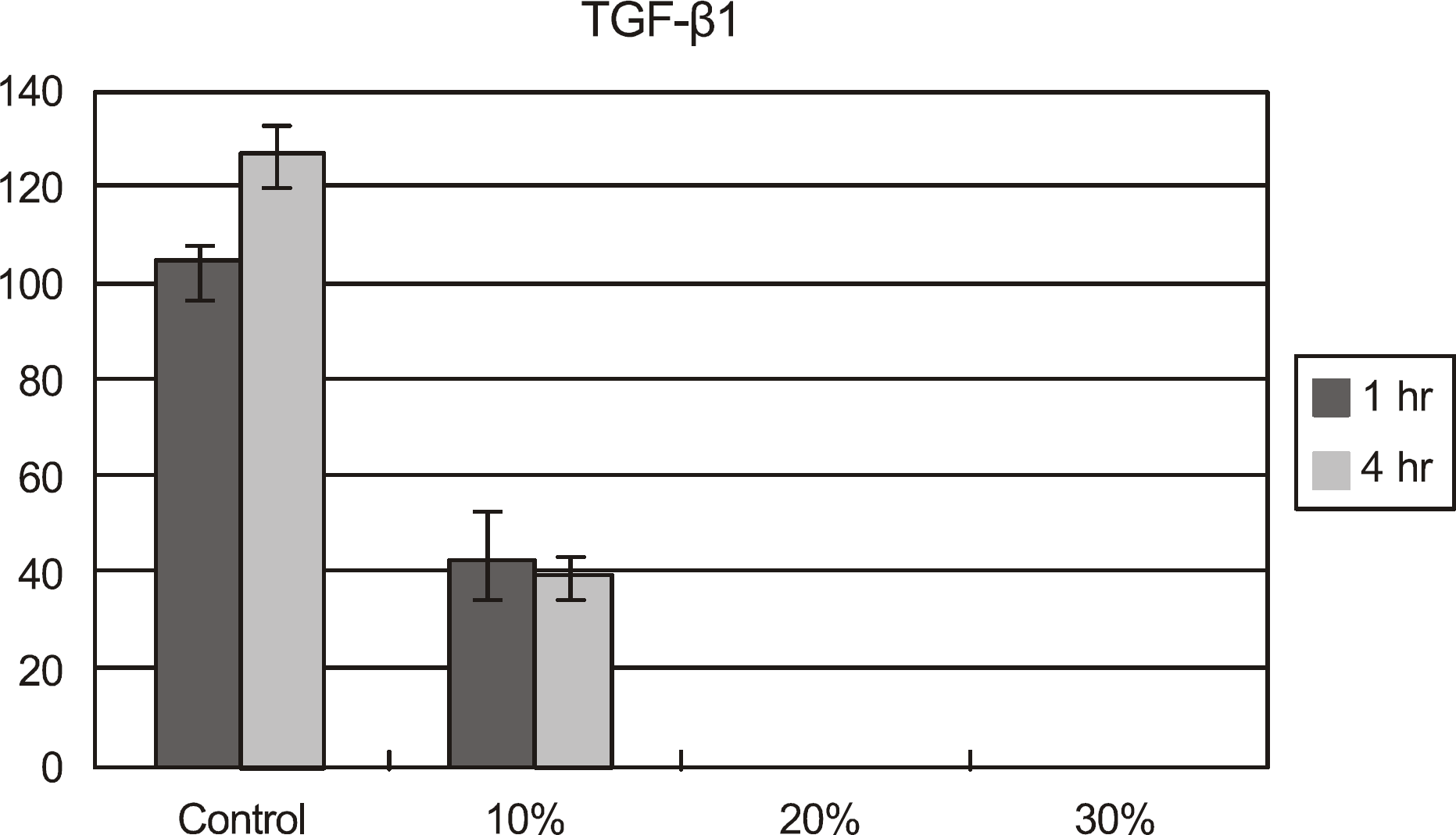 | Figure 4.TGF-β1 concentration of cultured corneal epithelial cells after exposed to calf serum. The concentration of TGF-β1 was declined significantly after exposed to all the concentrations of calf serum for up to 4 hours. Especially, TGF-β1 was not detected after exposed to 20, 30 % concentration of calf serum. |
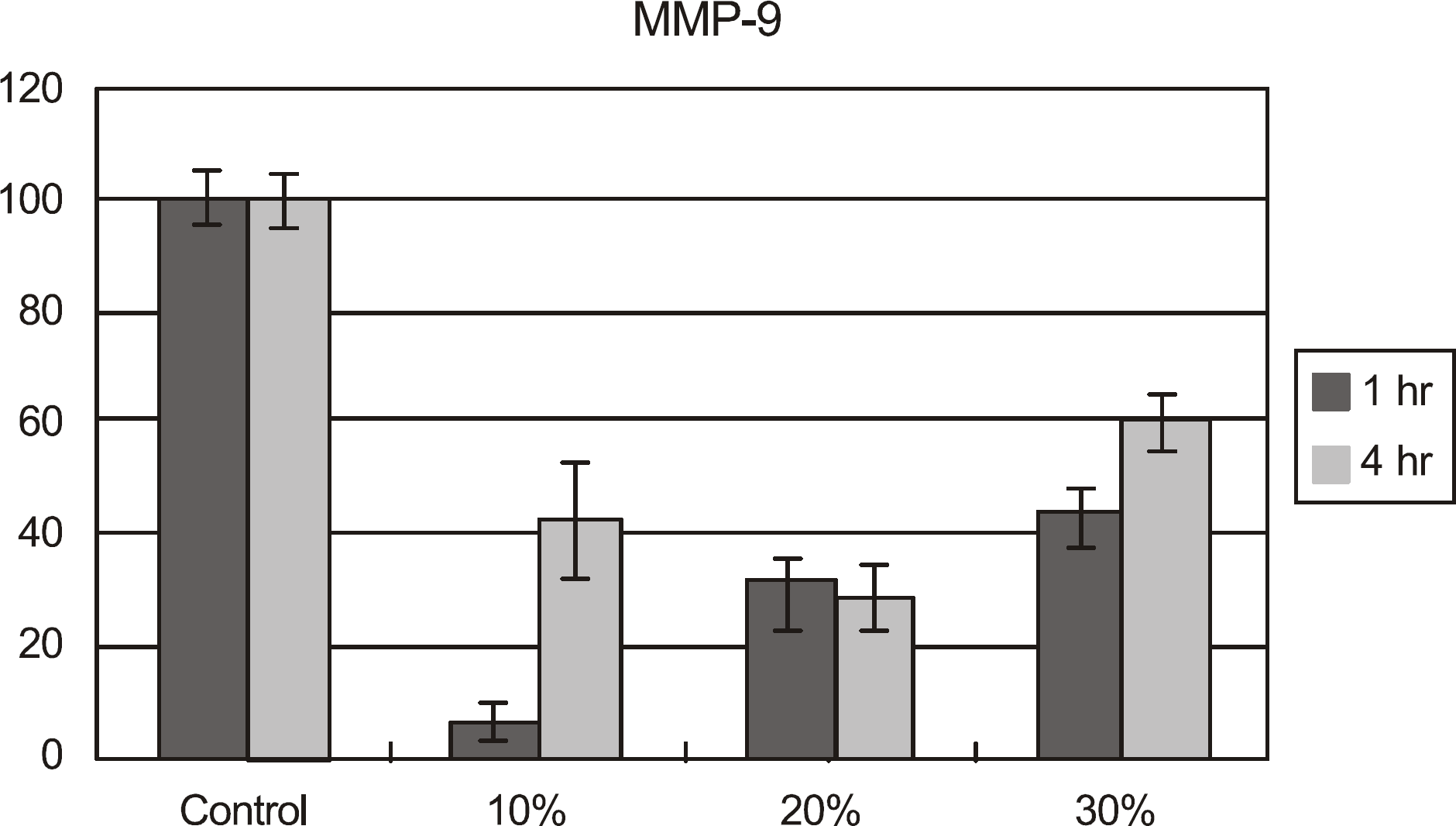 | Figure 5.MMP-9 concentration of cultured corneal epithelial cells after exposed to calf serum. The concentration of MMP-9 was lower than in the control after exposed to all the concentrations of calf serum for up to 4 hours. |
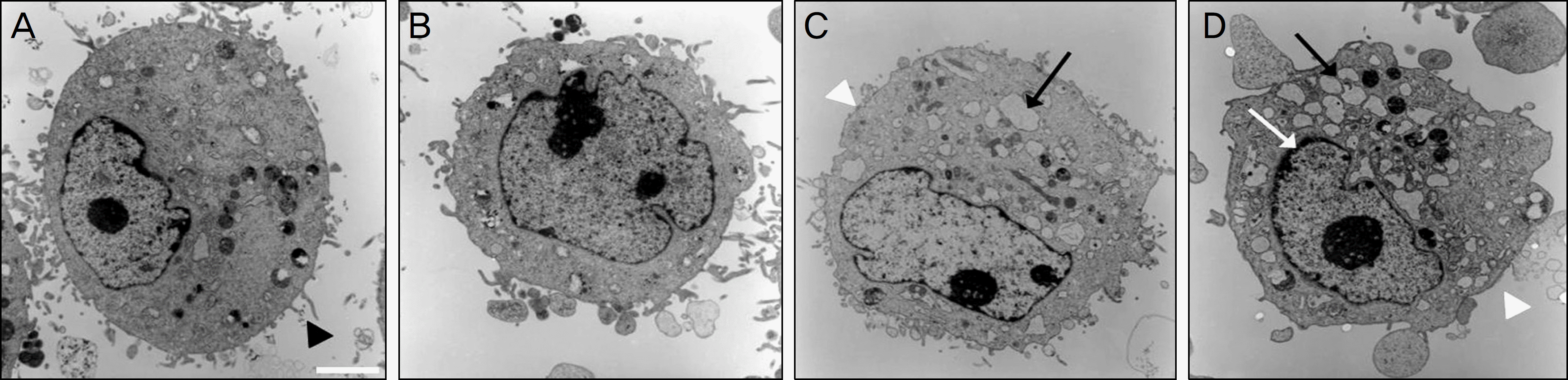 | Figure 6.Transmission electron micrographs of corneal epithelial cells appeared after 4-hour exposure to (A) control, (B) 10% calf serum, (C) 20% calf serum, and (D) 30% calf serum. (bar length 2 um, original magnification, ×2000–4000). In general, the plasma membrane with microvilli (black arrow head), nuclear membrane, and nuclei of corneal epithelial cells were visible. 30% calf serum had more severe and damaged cellular structures, such as the plasma membranes with microvilli being disrupted (white arrow head), well-developed vacuole formation (black arrow), and chromatin margination of the nucleus (white arrow), rather than 10, 20% calf serum. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download