Abstract
Purpose
To evaluate visual field (VF) changes in patients with pituitary adenoma after surgical treatment.
Methods
The present study retrospectively evaluated 96 eyes of 48 patients with pituitary adenoma who received surgical tumor removal between July 2001 and February 2010. Preoperative and postoperative clinical data including age, tumor volume, logMAR BCVA, surgical technique (transsphenoidal surgery and transcranial surgery), static perimetry scores (mean deviation [MD], pattern standard deviation [PSD], and visual field defect [VFD] scores) were reviewed.
Results
The MD (15.79%, p = 0.001) and PSD (3.98%, p = 0.003) improved postoperatively (mean postoperative fol-low-up period 1.85 months). Transsphenoidal surgery for tumor removal showed significant MD (26.99%, p = 0.000) and PSD (12.92%, p = 0.003) improvements. A multivariate regression analysis of the transsphenoidal surgery patient group revealed that the preoperative MD was related to the postoperative MD (Pearson = 0.762, p = 0.000), but negatively correlated to the amount of postoperative improvement in MD score (Pearson = −0.231, p = 0.046). Transcranial surgery did not significantly improve the MD (p = 0.419), PSD (p = 0.562), VFD score (p = 0.135), or logMAR BCVA (p = 0.708).
Conclusions
Visual filed defects in patients with pituitary adenoma improved after neurosurgical treatment. Better postoperative visual field outcomes were achieved in patients who had smaller preoperative visual field defects. Transsphenoidal surgery significantly improved the visual field defects and visual acuity in patients with non-functioning pituitary adenoma, compared to the transcranial surgery patients.
References
1. Kerrison JB, Lynn MJ, Baer CA, et al. Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol. 2000; 130:813–20.

2. Laws ER Jr, Trautmann JC, Hollenhorst RW Jr. Transsphenoidal decompression of the optic nerve and chiasm. Visual results in 62 patients. J Neurosurg. 1977; 46:717–22.
3. Powell M. Recovery of vision following transsphenoidal surgery for pituitary adenomas. Br J Neurosurg. 1995; 9:367–73.

4. Svien HJ, Love JG, Kennedy WC, et al. Status of vision following surgical treatment for pituitary chromophobe adenoma. J Neurosurg. 1965; 22:47–52.

5. Ciric I, Mikhael M, Stafford T, et al. Transsphenoidal microsurgery of pituitary macroadenomas with long-term follow-up results. J Neurosurg. 1983; 59:395–401.

6. Findlay G, McFadzean RM, Teasdale G. Recovery of vision following treatment of pituitary tumours: application of a new system of visual assessment. Trans Ophthalmol Soc U K. 1983; 103:212–6.
7. Lennerstrand G. Visual recovery after treatment for pituitary adenoma. Acta Ophthalmol. 1983; 61:1104–17.

8. Gnanalingham KK, Bhattacharjee S, Pennington R, et al. The time course of visual field recovery following transphenoidal surgery for pituitary adenomas: predictive factors for a good outcome. J Neurol Neurosurg Psychiatry. 2005; 76:415–9.

9. Cohen AR, Cooper PR, Kupersmith MJ, et al. Visual recovery after transsphenoidal removal of pituitary adenomas. Neurosurgery. 1985; 17:446–52.

10. Ogden TE. Nerve fiber layer of the primate retina: thickness and glial content. Vision Res. 1983; 23:581–7.

11. Blamires TL, Reeves BC. Vision defects in patients with peri-chiasmal lesions. Optom Vis Sci. 1996; 73:572–8.

12. Hirai T, Ito Y, Arai M, et al. Loss of stereopsis with optic chiasmal lesions and stereoscopic tests as a differential test. Ophthalmology. 2002; 109:1692–702.

13. Symon L, Jakubowski J. Transcranial management of pituitary tumours with suprasellar extension. J Neurol Neurosurg Psychiatry. 1979; 42:123–33.

14. Wang H, Sun W, Fu Z, et al. The pattern of visual impairment in patients with pituitary adenoma. J Int Med Res. 2008; 36:1064–9.

15. Thomas R, Shenoy K, Seshadri MS, et al. Visual field defects in non-functioning pituitary adenomas. Indian J Ophthalmol. 2002; 50:127–30.
16. Feinsod M, Selhorst JB, Hoyt WF, Wilson CB. Monitoring optic nerve function during craniotomy. J Neurosurg. 1976; 44:29–31.

17. Smith EJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979; 280:395–6.

18. Seddon HJ, Medawar PB, Smith H. Rate of regeneration of peripheral nerves in man. J Physiol. 1943; 102:191–215.

Figure 1.
Comparisons of preoperative and postoperative mean deviation. Mean deviations were improved in all patient group and statistically significant, except TCA group. TSA = transsphenoidal approach; TCA = transcranial approach. Better eye = better seeing eye; Worse eye = worse seeing eye. * p < 0.05; † p < 0.01; ‡ p < 0.001.
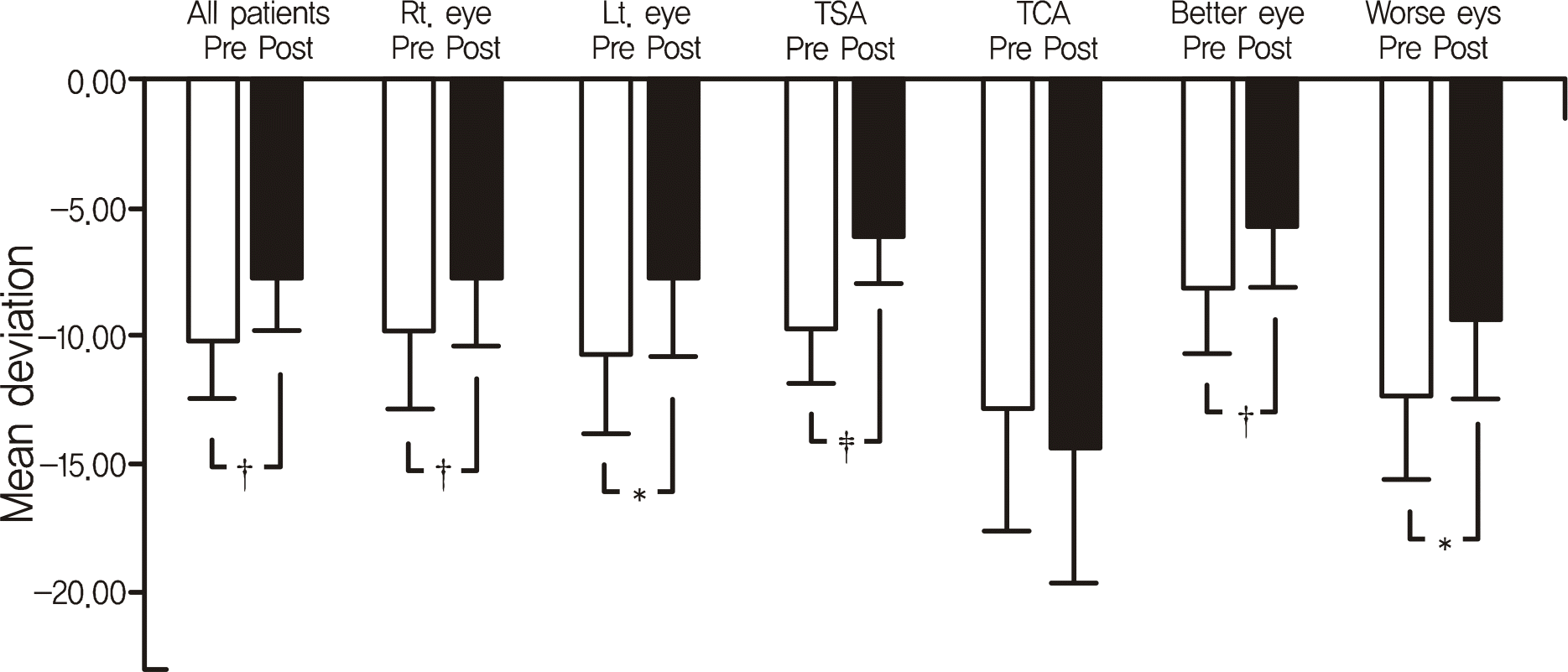
Figure 2.
Comparisons of preoperative and postoperative pattern standard deviations. Pattern standard deviations were improved in all patient group and statistically significant in all patient, Lt. eye, TSA, Better eye group. TSA = transsphenoidal approach; TCA = transcranial approach. Better eye = better seeing eye; Worse eye = worse seeing eye. * p < 0.05; † p < 0.01.
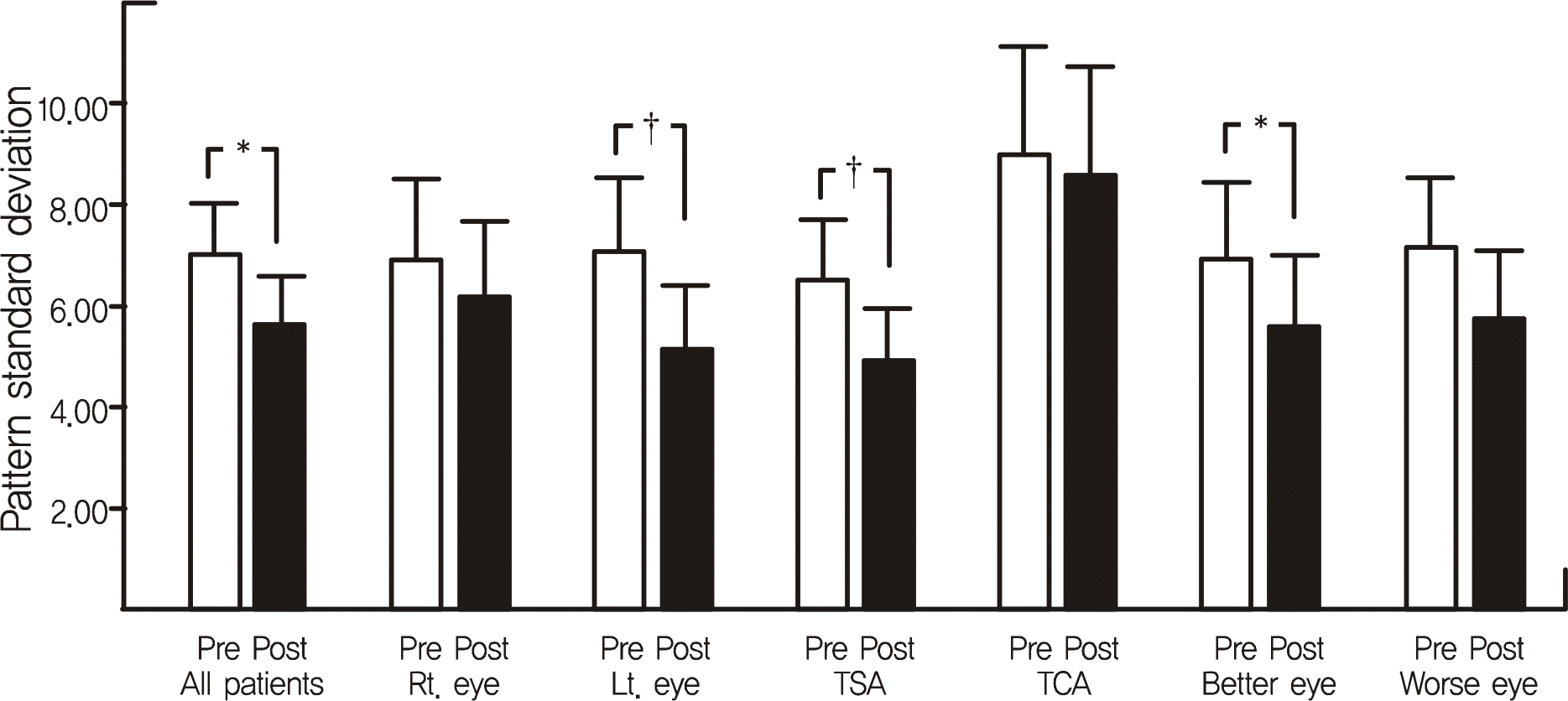
Figure 3.
Comparisons of preoperative and postoperative mean deviation (MD) and pattern standard deviation (PSD) in all patients. Preoperative MD correlate with postoperative MD significantly (Pearson = 0.733, p = 0.000). Preoperative PSD correlate with postoperative PSD significantly (Pearson = 0.602, p = 0.000).
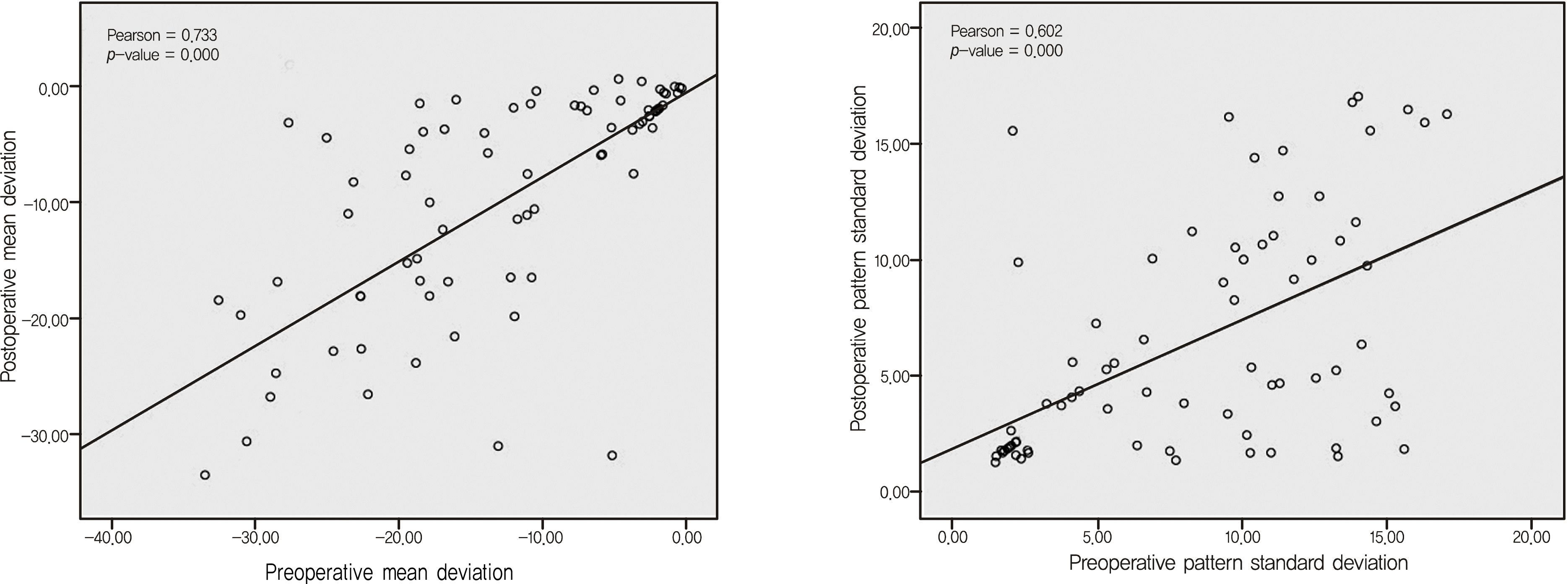
Figure 4.
Comparisons of preoperative mean deviation (MD) and postoperative relative changes of MD. (A) All patient group, (B) Transsphenoidal approach group, (C) Transcranial approach group. Relative change = (preoperative value – postoperative value) /preoperative value (% presentation). Preoperative MD has negative correlation with postoperative relative changes of MD and statistically significant in transsphenoidal approach group.
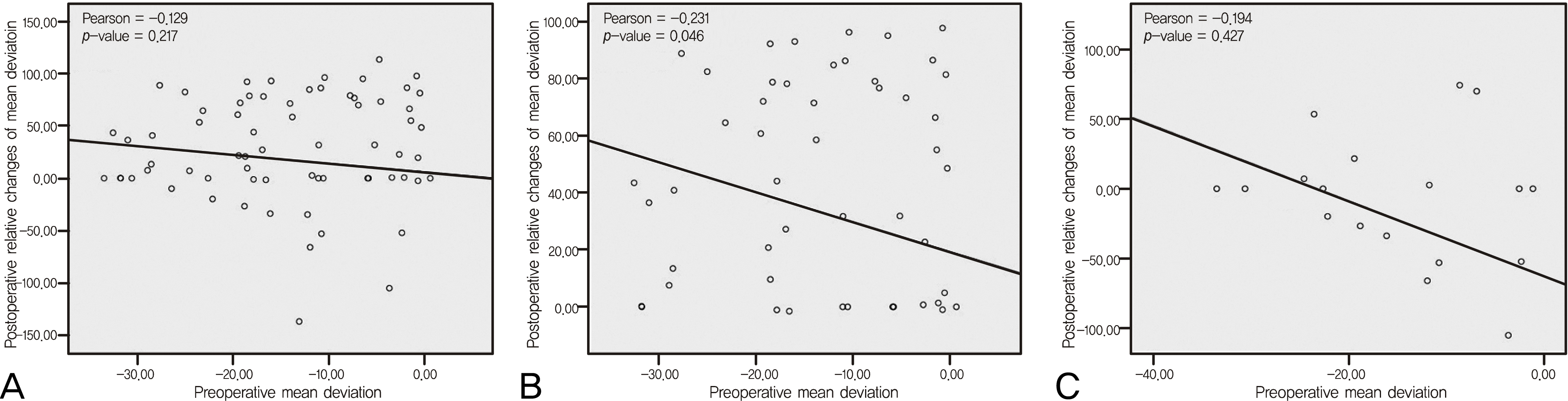
Table 1.
Visual field defect scores
Table 2.
Demographic characteristics of all patients
Table 3.
Comparisons of preoperative and postoperative visual field and logMAR visual acuity
| Variable | All patients | Rt. eye | Lt. eye | p-value | TSA | TCA | p-value | Better eye | Worse eye | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 96 | 48 | 48 | 76 | 20 | 48 | 48 | |||
| Mean deviation (mean ± SD) | ||||||||||
| Preop | −10.23 ± 9.79 | −9.78 ± 9.83 | −10.67 ± 9.85 | 0.251 | −9.58 ± 9.90 | −12.83 ± 9.15 | 0.246 | −8.11 ± 8.32 | −12.21 ± 10.68 | *0.039 |
| Postop | −7.73 ± 9.30 | −7.57 ± 9.15 | −7.88 ± 9.53 | 0.902 | −6.06 ± 8.18 | −14.31 ± 10.72 | †††0.000 | −5.69 ± 7.55 | −9.39 ± 10.67 | *0.039 |
| Changes | 2.50 ± 6.99 | 2.21 ± 4.90 | 2.78 ± 8.57 | 3.51 ± 6.44 | −1.78 ± 7.79 | 2.41 ± 5.06 | 2.81 ± 8.60 | |||
| Relative‡ | 15.79 ± 71.14 | 20.57 ± 39.84 | 11.22 ± 13.27 | 0.393 | 26.99 ± 41.12 | −28.40 ± 29.57 | ††0.002 | 22.09 ± 41.70 | 6.88 ± 13.54 | 0.300 |
| p-value | **0.001 | **0.004 | *0.029 | ***0.000 | 0.419 | **0.002 | *0.028 | |||
| Pattern standard deviation (mean ± SD) | ||||||||||
| Preop | 7.00 ± 4.92 | 6.94 ± 4.94 | 7.06 ± 4.96 | 0.902 | 6.50 ± 4.95 | 8.99 ± 4.39 | †0.046 | 6.92 ± 5.12 | 7.08 ± 4.77 | 0.956 |
| Postop | 5.62 ± 4.75 | 6.10 ± 5.13 | 5.16 ± 4.35 | 0.067 | 4.86 ± 4.51 | 8.62 ± 4.55 | ††0.002 | 5.46 ± 4.89 | 5.77 ± 4.65 | 0.770 |
| Changes | −1.38 ± 4.31 | −0.84 ± 4.58 | −1.89 ± 4.02 | −1.63 ± 4.61 | −0.36 ± 2.72 | −1.45 ± 4.23 | −1.30 ± 4.44 | |||
| Relative‡ | 3.98 ± 85.60 | −5.26 ± 104.64 | −12.84 ± 62.07 | 0.369 | 12.92 ± 6.35 | 4.09 ± 30.51 | 0.995 | 12.02 ± 35.30 | 6.33 ± 17.19 | 0.299 |
| p-value | **0.003 | 0.216 | **0.002 | **0.003 | 0.562 | *0.022 | 0.050 | |||
| Visual field defect area score (mean ± SD) | ||||||||||
| Preop | 1.35 ± 1.20 | 1.23 ± 1.21 | 1.45 ± 1.20 | 0.067 | 1.24 ± 1.23 | 1.78 ± 0.97 | 0.092 | 1.21 ± 1.14 | 1.55 ± 1.26 | 0.237 |
| Postop | 1.04 ± 1.15 | 1.00 ± 1.15 | 1.08 ± 1.16 | 0.359 | 0.78 ± 1.01 | 2.05 ± 1.12 | †††0.000 | 0.93 ± 1.07 | 1.21 ± 1.24 | 0.268 |
| Changes | −0.30 ± 0.81 | −0.23 ± 0.79 | −0.37 ± 0.84 | −0.45 ± 0.77 | 0.26 ± 0.73 | −0.27 ± 0.79 | −0.34 ± 0.84 | |||
| p-value | ***0.000 | *0.047 | **0.003 | ***0.000 | 0.135 | *0.022 | **0.008 | |||
| logMAR BCVA (mean ± SD) | ||||||||||
| Preop | 0.34 ± 0.49 | 0.35 ± 0.56 | 0.33 ± 0.43 | 0.949 | 0.31 ± 0.46 | 0.45 ± 0.63 | 0.354 | 0.22 ± 0.37 | 0.48 ± 0.61 | *0.027 |
| Postop | 0.28 ± 0.48 | 0.30 ± 0.54 | 0.25 ± 0.41 | 0.877 | 0.22 ± 0.41 | 0.49 ± 0.66 | †0.03 | 0.18 ± 0.34 | 0.39 ± 0.62 | 0.072 |
| Changes | −0.06 ± 0.41 | −0.04 ± 0.41 | −0.07 ± 0.41 | −0.09 ± 0.38 | 0.04 ± 0.51 | −0.03 ± 0.37 | −0.08 ± 0.45 | |||
| p-value | 0.146 | 0.444 | 0.203 | *0.046 | 0.708 | 0.509 | 0.185 | |||
Table 4.
Relationship between preoperative MD, PSD and visual outcomes
| MD.change | PSD.change | PostOp.MD | PostOp.PSD | ||
|---|---|---|---|---|---|
| All patient | |||||
| PreOp.MD | Pearson | −0.129 | *0.267 | ‡0.733 | ‡-0.602 |
| p-value | 0.217 | 0.009 | 0.000 | 0.000 | |
| PreOp.PSD | Pearson | 0.073 | †0.273 | ‡-0.356 | ‡0.602 |
| p-value | 0.482 | 0.008 | 0.000 | 0.000 | |
| Transsphenoidal approach | |||||
| PreOp.MD | Pearson | *-0.231 | *0.294 | ‡0.762 | ‡-0.589 |
| p-value | 0.046 | 0.010 | 0.000 | 0.000 | |
| PreOp.PSD | Pearson | ‡0.316 | †0.298 | †-0.306 | ‡0.529 |
| p-value | 0.006 | 0.009 | 0.008 | 0.000 | |
| Transcranial approach | |||||
| PreOp.MD | Pearson | −0.194 | 0.076 | †0.703 | †-0.628 |
| p-value | 0.427 | 0.756 | 0.001 | 0.004 | |
| PreOp.PSD | Pearson | −0.028 | 0.147 | *-0.544 | ‡0.816 |
| p-value | 0.910 | 0.548 | 0.016 | 0.000 | |
Preop MD = preoperative mean deviation; Preop PSD = preoperative pattern standard deviation; Postop MD = postoperative mean deviation; Postop PSD = preoperative pattern standard deviation; MD change = postoperative relative changes of mean deviation; PSD change = postoperative relative changes of pattern standard deviation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download