Abstract
Purpose
To evaluate the protective effect of creatine on the survival of retinal ganglion cells after serum deprivation.
Methods
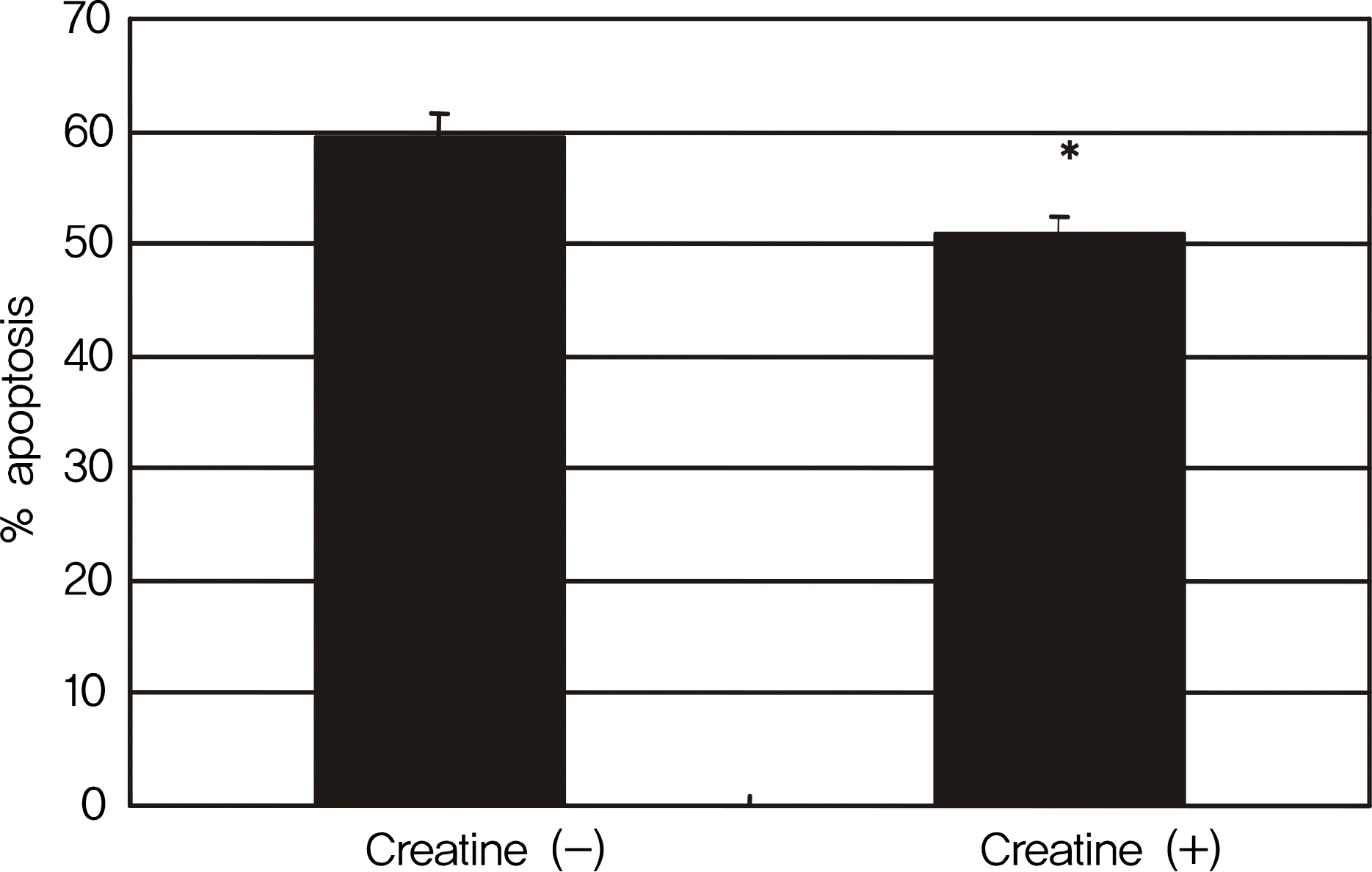
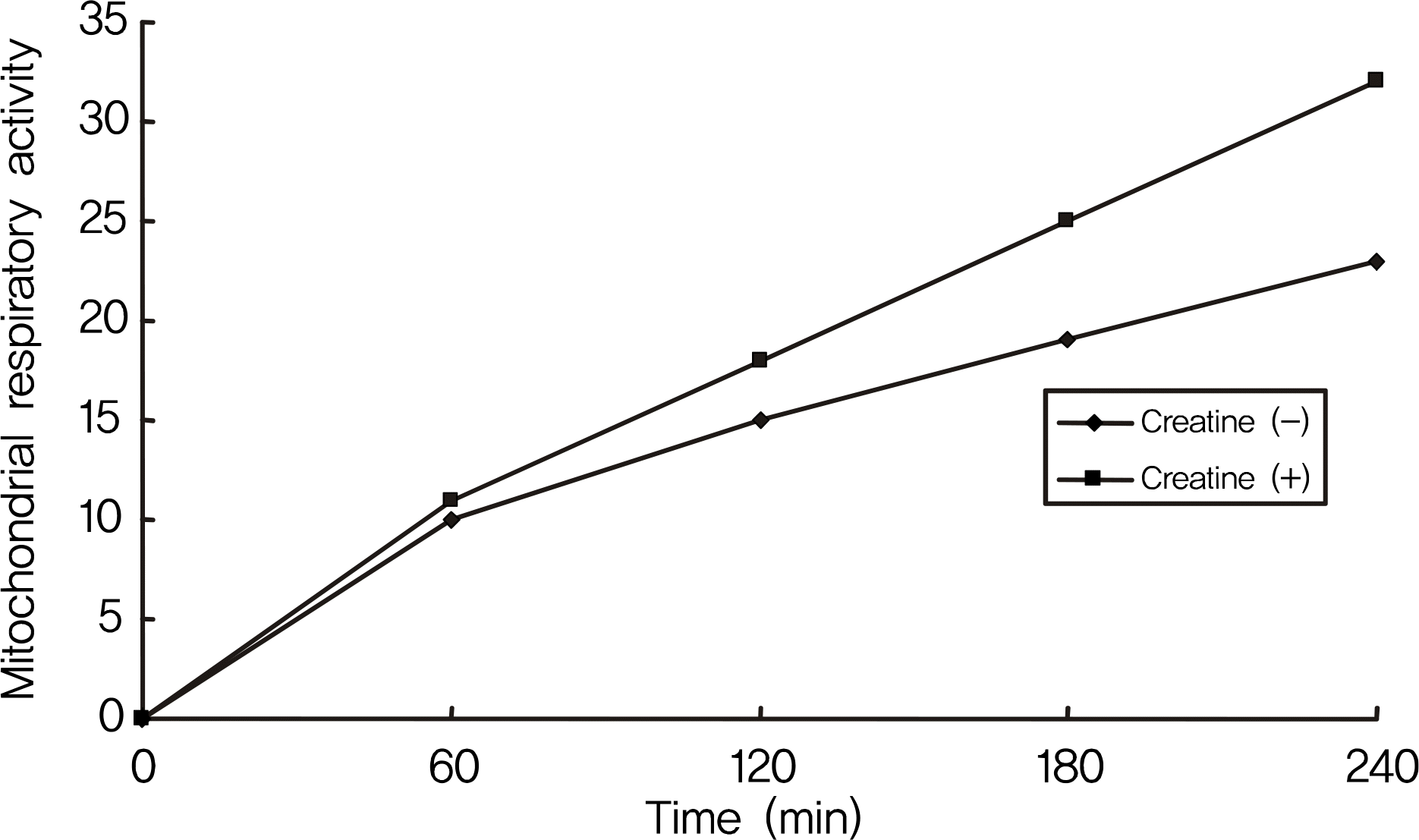
RGC-5 cells were exposed to 5 mM creatine with serum-free media for 4 days. Cellular survival and mitochondrial respiratory activity were measured with MTT assay and resazurin assay, respectively. Degree of apoptosis was evaluated with vital staining using acridine orange/Hoechest 33342 and flow cytometric analysis using annexin/PI, respectively.
References
1. Ames A 3rd. CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000; 34:42–68.

2. Holtzman D, Olson JE, Nguyen H, et al. Brain cellular and mitochondrial respiration in media of altered pH. Metab Brain Dis. 1987; 2:127–37.

3. Hemmer W, Wallimann T. Functional aspects of creatine kinase in brain. Dev Neurosci. 1993; 15:249–60.

4. Wallimann T, Schnyder T, Schlegel J, et al. Subcellular compart-mentation of creatine kinase isoenzymes, regulation of CK and oc-tameric structure of mitochondrial CK: important aspects of the phosphoryl-creatine circuit. Prog Clin Biol Res. 1989; 315:159–76.
5. Wallimann T, Wyss M, Brdiczka D, et al. Intracellular compart-mentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992; 281:21–40.

6. McNaught KS, Carrupt PA, Altomare C, et al. Isoquinoline de-rivatives as endogenous neurotoxins in the aetiology of Parkinson's disease. Biochem Pharmacol. 1998; 56:921–33.

7. Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem. 2000; 74:1968–78.
8. Michaelis T, Wick M, Fujimori H, et al. Proton MRS of oral creatine supplementation in rats. Cerebral metabolite concentrations and ischemic challenge. NMR Biomed. 1999; 12:309–14.

9. Wilken B, Ramirez JM, Probst I, et al. Creatine protects the central respiratory network of mammals under anoxic conditions. Pediatr Res. 1998; 43:8–14.

10. Wilken B, Ramirez JM, Probst I, et al. Anoxic ATP depletion in neonatal mice brainstem is prevented by creatine supplementation. Arch Arch Dis Child Fetal Neonatal Ed. 2000; 82:F224–7.

11. Zhu S, Li M, Figueroa BE, et al. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neurosci. 2004; 24:5909–12.

12. Adcock KH, Nedelcu J, Loenneker T, et al. Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hy-poxia-ischemia. Dev Neurosci. 2002; 24:382–8.

13. Chen L, Roberts R, Friedman DL. Expression of brain-type creatine kinase and ubiquitous mitochondrial creatine kinase in the fetal rat brain: evidence for a nuclear energy shuttle. J Comp Neurol. 1995; 363:389–401.

14. Hemmer W, Riesinger I, Wallimann T, et al. Brain-type creatine kinase in photoreceptor cell outer segments: role of a phosphocreatine circuit in outer segment energy metabolism and phototransduction. J Cell Sci. 1993; 106:671–83.

15. Dolder M, Walzel B, Speer O, et al. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003; 278:17760–6.
16. O'Gorman E, Beutner G, Dolder M, et al. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997; 414:253–7.
17. Acosta ML, Kalloniatis M, Christie DL. Creatine transporter localization in developing and adult retina: importance of creatine to retinal function. Am J Physiol Cell Physiol. 2005; 289:C1015–23.

18. Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001; 86:1–12.
19. Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989; 119:203–10.

20. Mpoke SS, Wolfe J. Differential staining of apoptotic nuclei in liv-ing cells: application to macronuclear elimination in Tetrahymena. J Histochem Cytochem. 1997; 45:675–83.
21. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995; 184:39–51.

22. Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008; 173:339–52.

23. Abu-Amero KK, Bosley TM. Detection of mitochondrial respiratory dysfunction in circulating lymphocytes using resazurin. Arch Pathol Lab Med. 2005; 129:1295–8.

24. Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem. 1994; 133:193–220.

25. Wallimann T, Wyss M, Brdiczka D, et al. Intracellular compart-mentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992; 281:21–40.

26. Whittingham TS, Lipton P. Cerebral synaptic transmission during anoxia is protected by creatine. J Neurochem. 1981; 37:1618–21.

27. Carter AJ, Müller RE, Pschorn U, Stransky W. Preincubation with creatine enhances levels of creatine phosphate and prevents anoxic damage in rat hippocampal slices. J Neurochem. 1995; 64:2691–9.

28. Chung I, Zhang Y, Eubanks JH, Zhang L. Attenuation of hypoxic current by intracellular applications of ATP regenerating agents in hippocampal CA1 neurons of rat brain slices. Neuroscience. 1998; 86:1101–7.

29. Lemasters JJ, Qian T, Bradham CA, et al. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr. 1999; 31:305–19.
30. O’Gorman E, Beutner G, Dolder M, et al. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997; 414:253–7.
32. Wyss M, Smeitink J, Wevers RA, Wallimann T. Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta. 1992; 1102:119–66.

33. Passonneau JV, Lowry OH. Metabolite flux in single neurons during ischemia and anesthesia. Curr Probl Clin Biochem. 1971; 3:198–212.
34. Stachowiak O, Dolder M, Wallimann T, Richter C. Mitochondrial creatine kinase is a prime target of peroxynitrite-induced mod-ification and inactivation. J Biol Chem. 1998; 273:16694–9.

Figure 1.
Effect of creatine on the survival of RGC-5 cells after serum deprivation for 4 days. Addition of 5 mM creatine significantly increased cellular survival. * p < 0.05.
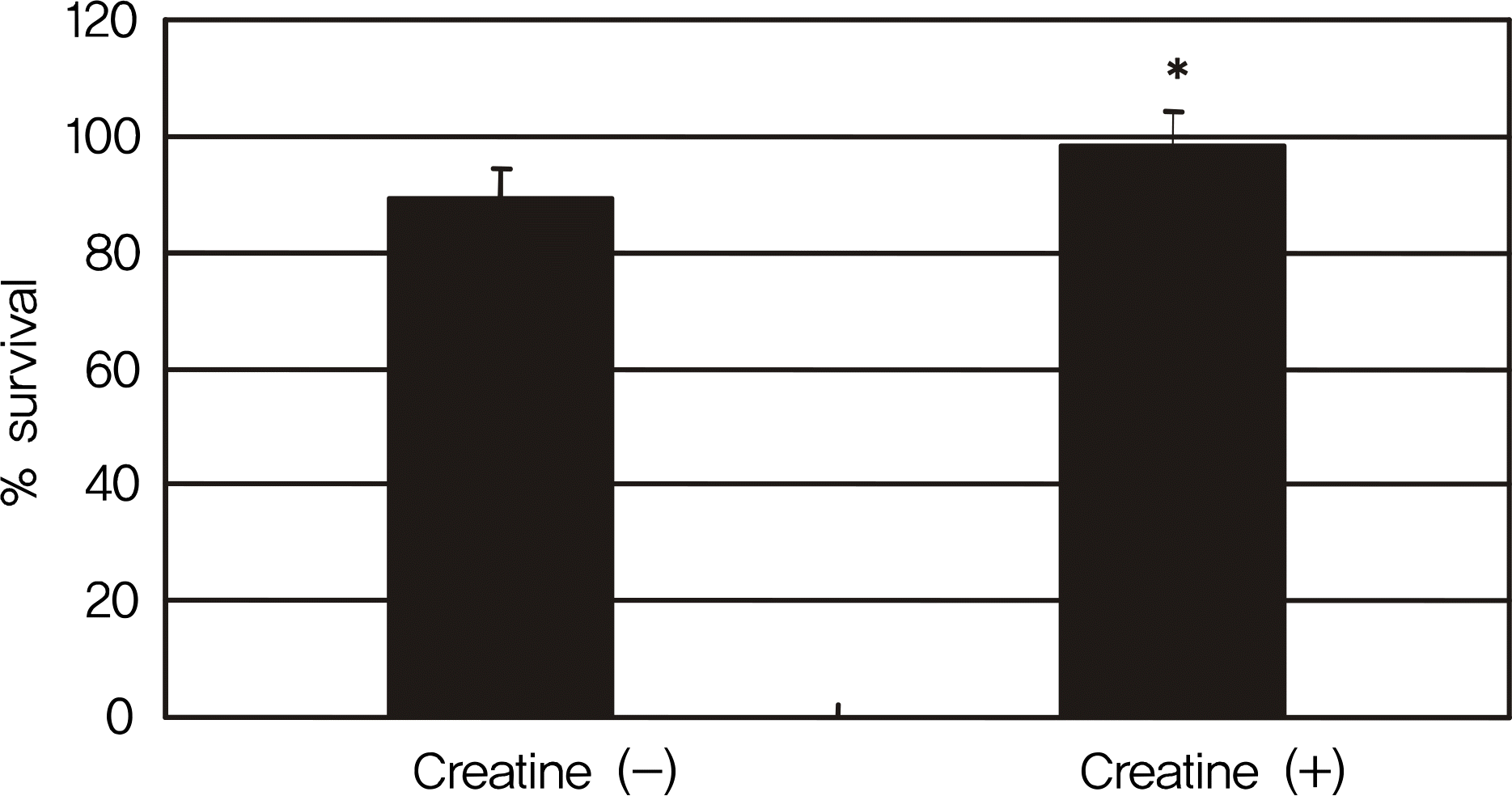
Figure 2.
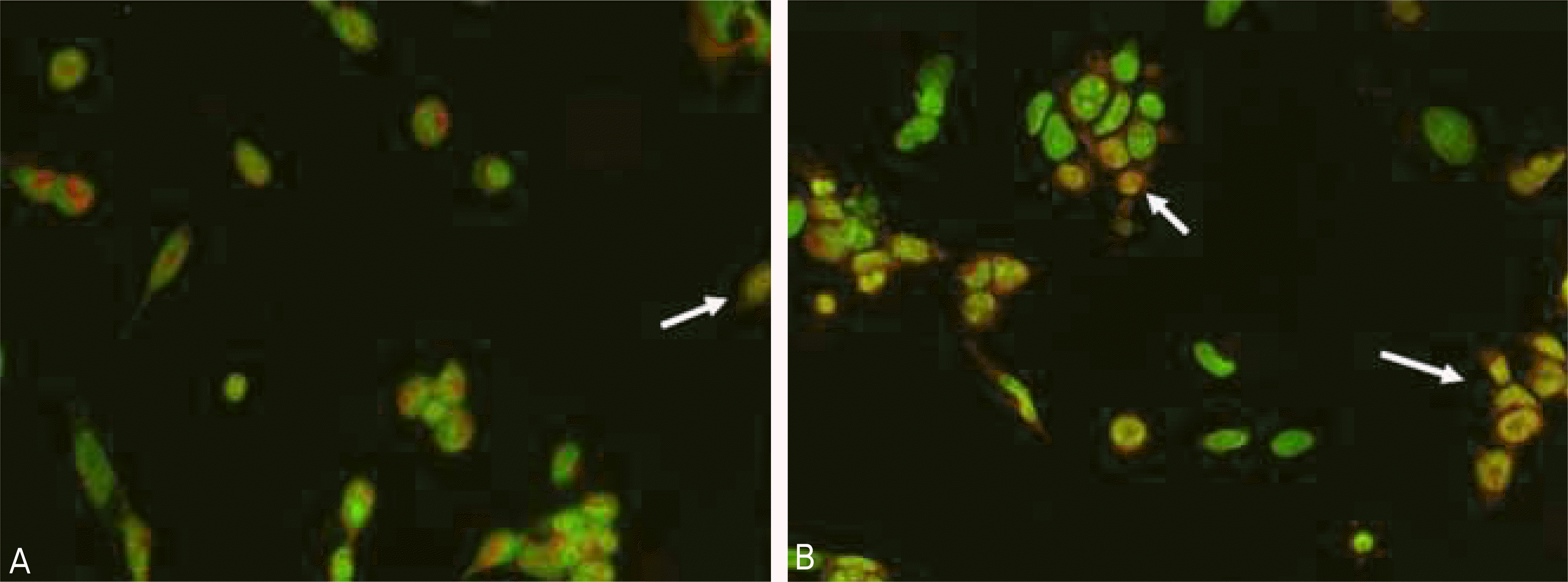
AO/HO double staining of RGC-5 cells with or without treatment of 5 mM creatine for 4 days. Normal control cell stains blue nuclei and brilliant red cytoplasm. In contrast, the apoptotic cell (arrows) shows yellow to orange-red nuclei, becoming indis-tinguishable from the cytoplasm. Addition of 5 mM creatine (A) revealed less apoptotic cells compared non-treated control (B) (×200).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download