Abstract
Purpose
To evaluate the expression of nerve growth factor (NGF) in the rat cornea and lacrimal gland before and after corneal epithelial wounding.
Methods
Twenty-nine Sprague-Dawley male rats were used in the present study. Corneal trephination was performed using a 4.0-mm diameter trephine before scratch and at 24, 48, 72 hours after debridement. The lacrimal gland was excised before scratch and at 24 hours after epithelial debridement. NGF levels of the excised cornea and lacrimal gland were measured in rat corneas by enzyme-linked immunosorbent assay (ELISA). Immunohistochemistry staining was performed on rat corneas and lacrimal glands.
Results
The NGF/total protein ratio (NGF/tP) increased after wounding in the cornea and lacrimal gland. NGF levels in the cornea significantly increased in the wounded group until the 2nd day after wounding (p < 0.05). After NGF concentration peaked on the 1st day, there was a progressive decline after wounding. Additionally, the NGF concentration in the lacrimal gland of the wounded group was significantly higher than that of the control group at 24 hours after epithelial debridement (p = 0.001). Immunohistochemistry staining showed that NGF staining was stronger in rat corneas and lacrimal glands after epithelial debridement than before.
Go to : 
References
2. Ebendal T. Function and evolution in the NGF family and its receptors. J Neurosci Res. 1992; 32:461–70.

3. Lambiase A, Rama P, Bonini S, et al. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998; 338:1174–80.

4. You L, Kruse FE, Völcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000; 41:692–702.
5. Lambiase A, Bonini S, Aloe L, et al. Anti-inflammatory and healing properties of nerve growth factor in immune corneal ulcers with stromal melting. Arch Ophthalmol. 2000; 118:1446–9.

6. Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006; 25:352–5.

7. Woo HM, Bentley E, Campbell SF, et al. Nerve growth factor and corneal wound healing in dogs. Exp Eye Res. 2005; 80:633–42.

8. Nguyen DH, Beuerman RW, Thompson HW, DiLoreto DA. Growth factor and neurotrophic factor mRNA in human lacrimal gland. Cornea. 1997; 16:192–9.

9. Lambiase A, Manni L, Bonini S, et al. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000; 41:1063–9.
10. Ghinelli E, Johansson J, Ríos JD, et al. Presence and localization of neurotrophins and neurotrophin receptors in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2003; 44:3352–7.

11. Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002; 43:987–94.
12. Kim SY, Choi JS, Joo CK. Effects of nicergoline on corneal epithelial wound healing in rat eyes. Invest Ophthalmol Vis Sci. 2009; 50:621–5.

13. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–9.
15. Dana MR, Hamrah P. Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye. Adv Exp Med Biol. 2002; 506:729–38.

16. Lyne A. Corneal sensitivity after surgery. Trans Ophthalmol Soc U K. 1982; 102:302–5.
17. Moilanen JA, Vesaluoma MH, Müller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003; 44:1064–9.

18. Joo MJ, Yuhan KR, Hyon JY, et al. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004; 122:1338–41.
Go to : 
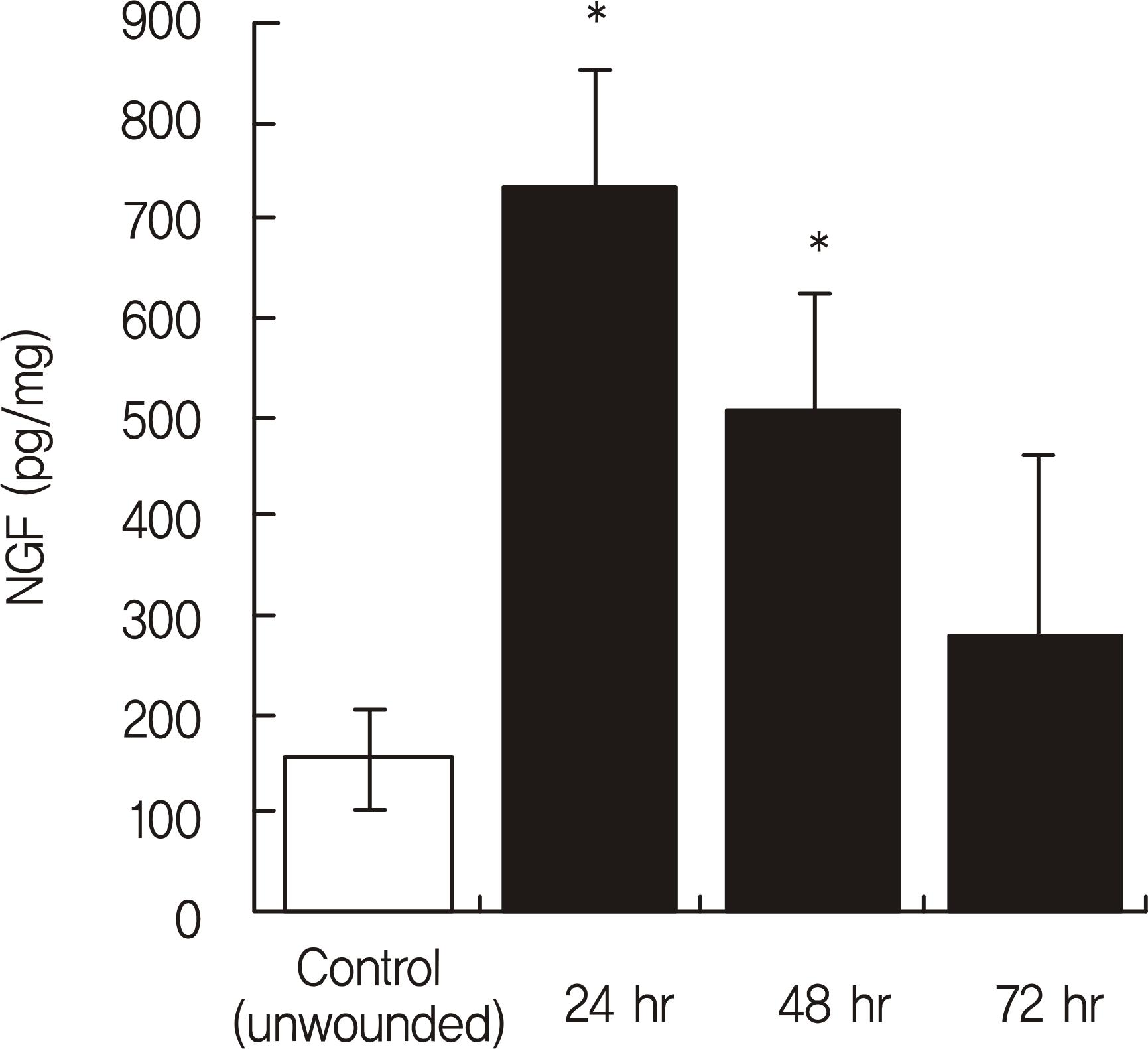 | Figure 1.The nerve growth factor/total protein (NGF/tP, pg/mg) concentrations of rat corneal tissue after epithelial debridement. NGF protein levels were quantified in the cornea over time after complete epithelium debridement. Five ani-mals were evaluated per time point. NGF levels of the corneal tissue significantly increased in the wounded group until 2 days after wounding. * p < 0.05, n = 5 per group, ANOVA repeated measure. |
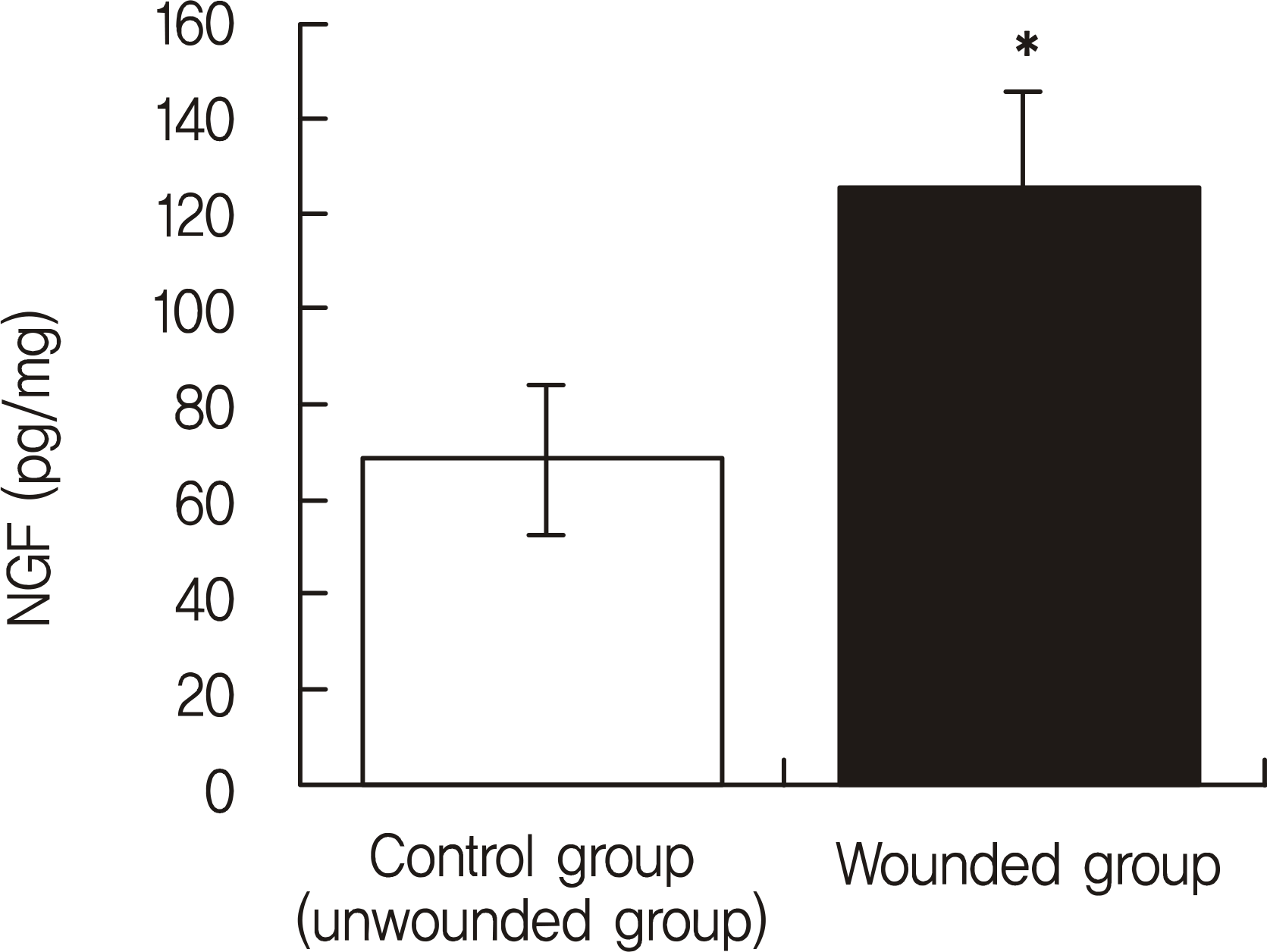 | Figure 2.The nerve growth factor/total protein (NGF/tP, pg/mg) concentrations of the rat lacrimal gland tissue. At 24 hours after epithelial debridement, the rat lacrimal gland NGF concentrations increased when compared with the control group. * p = 0.001, n = 5 per group, ANOVA repeated measure. |
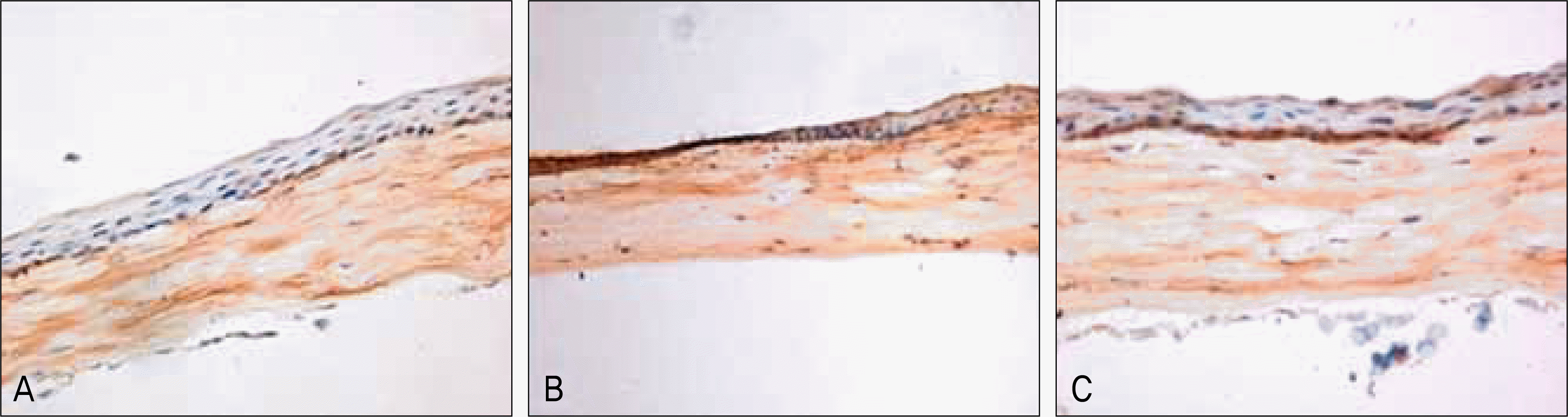 | Figure 3.Immunohistologic detection of NGF in rat corneal tissue of the unwounded, control group (A) and wounded group at 1 day (B) and 7 days (C) after epithelial debridement. NGF staining is stronger in the cornea at 24 hours after epithelial debridement than in unwounded cornea (Streptavidin-biotin, magnification ×200). |
 | Figure 4.Immunohistologic detection of NGF in rat lacrimal tissue of the unwounded, control group (A) and wounded group at 24 hours (B) after epithelial debridement. At 24 hours after epithelial debridement, the lacrimal NGF expression increased in the aci-nar cell and lumen when compared with that of control group (Streptavidin-biotin, magnification ×40). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download