Abstract
Purpose
To investigate the effects of an amniotic membrane patch on corneal epithelial thickness and formation of hemidesmosomes during corneal stromal wound healing.
Methods
A stromal wound 9 mm in diameter and 130 μm in depth was created on rabbit cornea using a microkeratome. The changes in corneal epithelial thickness and hemidesmosome formations were compared between the amniotic membrane, contact lens, and control groups. Changes in the corneal epithelium were examined using H&E staining and hemidesmosome formation was examined using an electron microscope at 2 and 4 weeks after flap removal.
Results
Two weeks after treatment, the corneal epithelial thickness was 95.3 ± 6.3 μm in the amniotic membrane group being significantly thicker than 76.4 ± 5.1 μm in the contact lens group and 68.3 ± 6.1 μm in the control group. Furthermore, more hemidesmosome formations were observed in the amniotic membrane group compared to the other 2 groups. However, there were no significant differences in corneal epithelial thickness or hemidesmosome formation among the 3 groups at week 4.
Go to : 
References
1. Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910; 15:307.
2. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995; 14:473–84.

3. Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000; 107:980–9.

4. Kruse FE, Rohrschneider K, Völcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999; 106:1504–10.

5. Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000; 84:826–33.

6. Kim JS, Kim JC, Hahn TW, Park WC. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. 2001; 20:720–6.

7. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001; 108:449–60.

8. Hanada K, Shimazaki J, Shimmura S, Tsubota K. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. Am J Ophthalmol. 2001; 131:324–31.

9. van Herendael BJ, Oberti C, Brosens I. Microanatomy of the human amniotic membranes. A light microscopic, transmission, and scanning electron microscopic study. Am J Obstet Gynecol. 1978; 131:872–80.
10. Dua HS, Azuara-Blanco A. Amniotic membrane transplantation. Br J Ophthalmol. 1999; 83:748–52.
11. Kim JC, Tseng SC. The effects on inhibition of corneal neo-vascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 1995; 9:32–46.

12. Kim SY. Korea External Rye Disease Society. Cornea. 2nd ed.Seoul: Ilchokak;2005. p. 2–3.
13. Kim YH, Lee DH, Lew HM. The comparison of the corneal epithelial healing according to treatment modalities in rabbit. J Korean Ophthalmol Soc. 1999; 40:2683–92.
14. Kang KM, Wee WR. The use if ascorbic acid after excimer laser photorefractive keratectomy in rabbits. J Korean Ophthalmol Soc. 1996; 37:1620–5.
15. Sohn JH, Choi SK, Lee JH. The effect of disposable bandage contact lenses on time and velocity of corneal epithelial healing after myopic epikeratoplasty. J Korean Ophthalmol Soc. 1995; 36:1422–8.
16. Fernandes M, Sridhar MS, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction. Cornea. 2005; 24:643–53.

17. Li H, Niederkorn JY, Neelam S, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005; 46:900–7.

18. Cooper LJ, Kinoshita S, German M, et al. An investigation into the composition of amniotic membrane used for ocular surface reconstruction. Cornea. 2005; 24:722–9.

19. Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005; 16:233–40.

20. Wilson SE, He YG, Weng J, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996; 62:325–7.

21. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999; 179:325–35.
22. Kim JC. A Practical Guide to Ocular Surface Disorders. 1st ed.Seoul: Naeweh Haksool;2006. p. 20–3.
23. Woo HM, Kim HS, Kweon OK, et al. Effects of amniotic membrane on epithelial wound healing and stromal remodeling after excimer laser keratectomy in rabbit cornea. Br J Ophthalmol. 2001; 85:345–9.
24. Yoshita T, Kobayashi A, Sugiyama K, Tseng SC. Oxygen permeability of amniotic membrane and actual tear oxygen tension beneath amniotic membrane patch. Am J Ophthalmol. 2004; 138:486–7.

25. Zagon IS, Sassani JW, Ruth TB, McLaughlin PJ. Epithelial adhesion complexes and organ culture of the human cornea. Brain Res. 2001; 900:205–13.

26. Xu YG, Choi SH, Ko SM, et al. Morphology and adhesion complex of cultured epithelium, on amniotic membrane in vitro and in vivo. J Korean Ophthalmol Soc. 2006; 47:160–70.
Go to : 
 | Figure 1.Photographs showing the making and removal of the corneal wound (diameter 9 mm, depth 130 μm). (A) Creating the corneal wound flap with Microkeratome (BDK3000, Becton & Dickinson, Franklin Lakes, NJ, USA). (B) Corneal flap. (C) Lifting of the corneal wound flap and cutting the hinge of the corneal flap using a blade. (D) Fluorescein dye staining after corneal flap removal. |
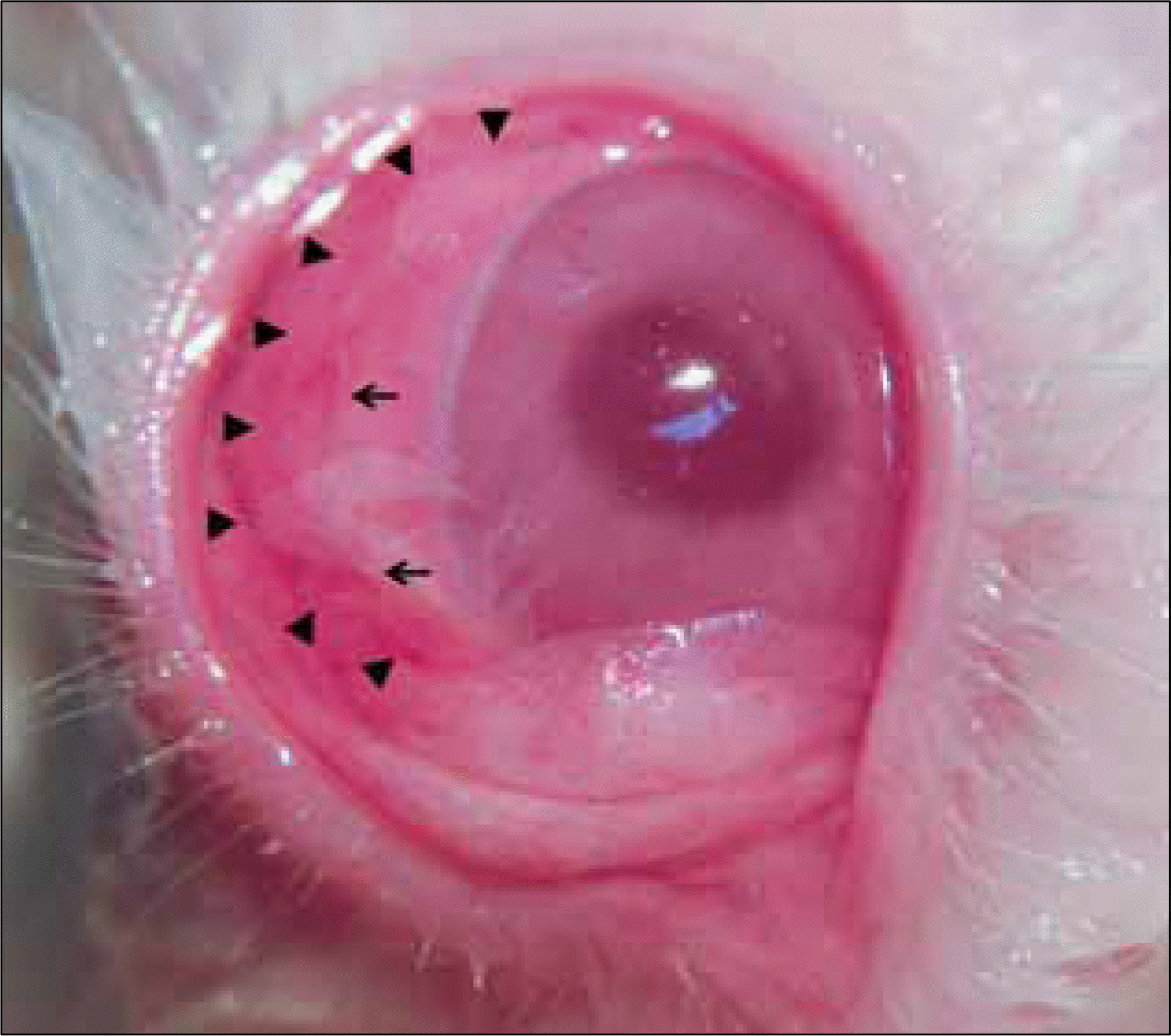 | Figure 2.Photograph of the rabbit cornea after amniotic membrane patch graft. Arrowheads indicate the original amniotic membrane patch margin (diameter 14 mm) and arrows indicate 10-0 nylon sutures. |
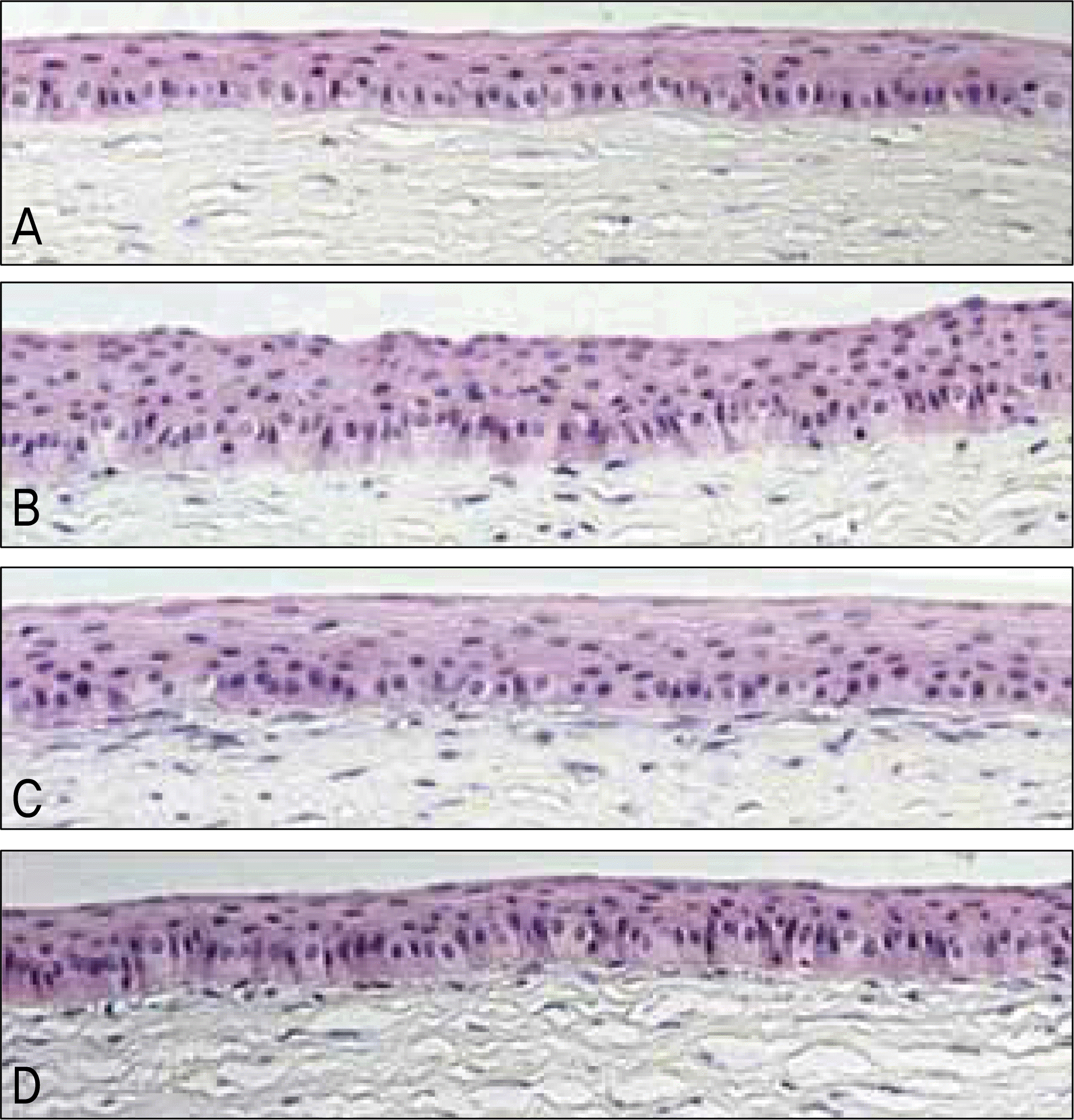 | Figure 3.Light microscopy of the central cornea at 2 weeks. (A) Normal cornea. (B) Amniotic membrane group. (C) Contact lens group. (D) Control group. Corneal epithelial thickness in the amniotic membrane group is thicker than the other groups (Hematoxylin-Eosin stain, original magnification ×100). |
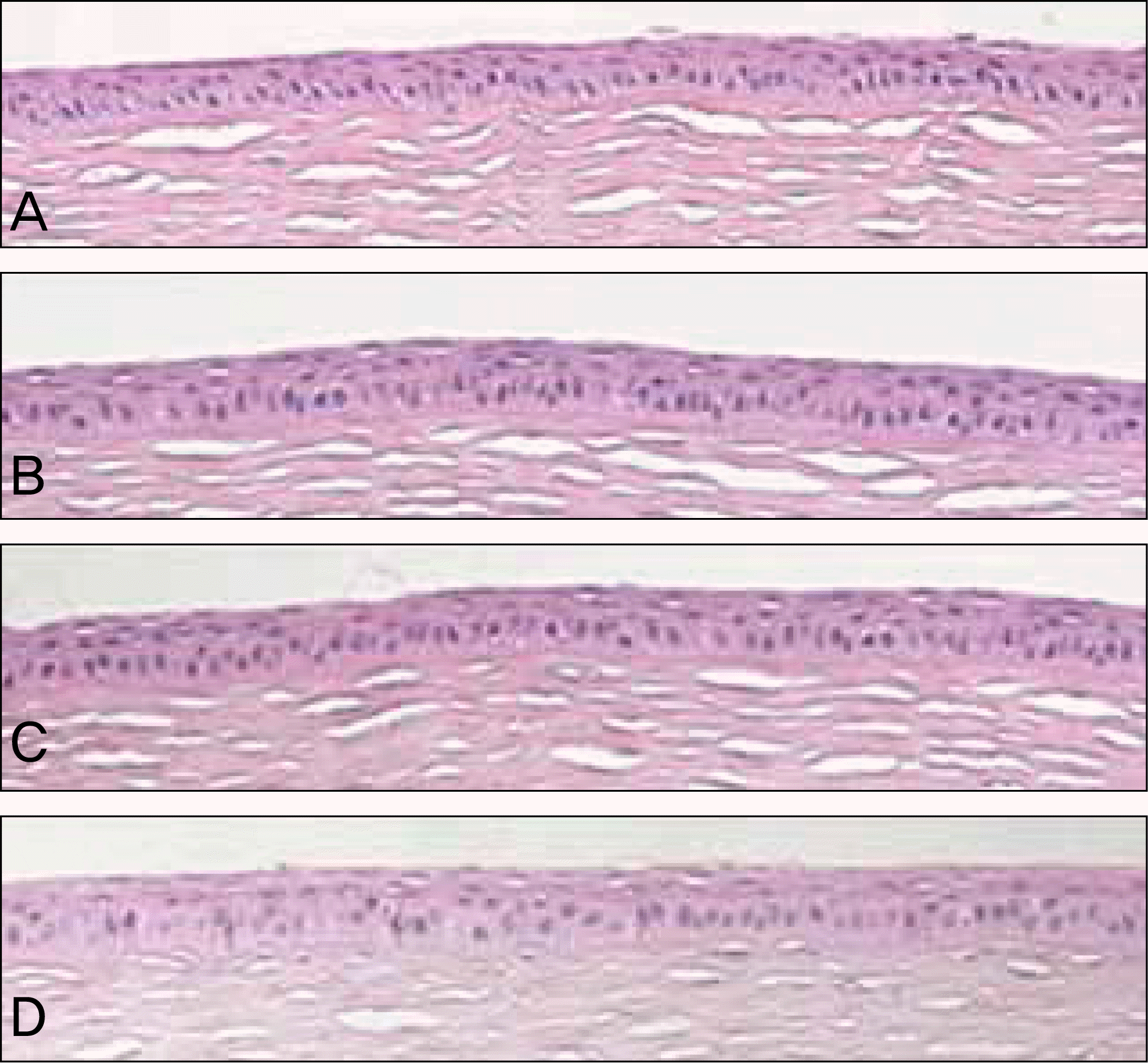 | Figure 4.Light microscopy of central cornea at 4 weeks. (A) Normal cornea. (B) Amniotic membrane group. (C) Contact lens group. (D) Control group. Corneal epithelial thickness is similar in all groups (Hematoxylin-Eosin stain, original magnification ×100). |
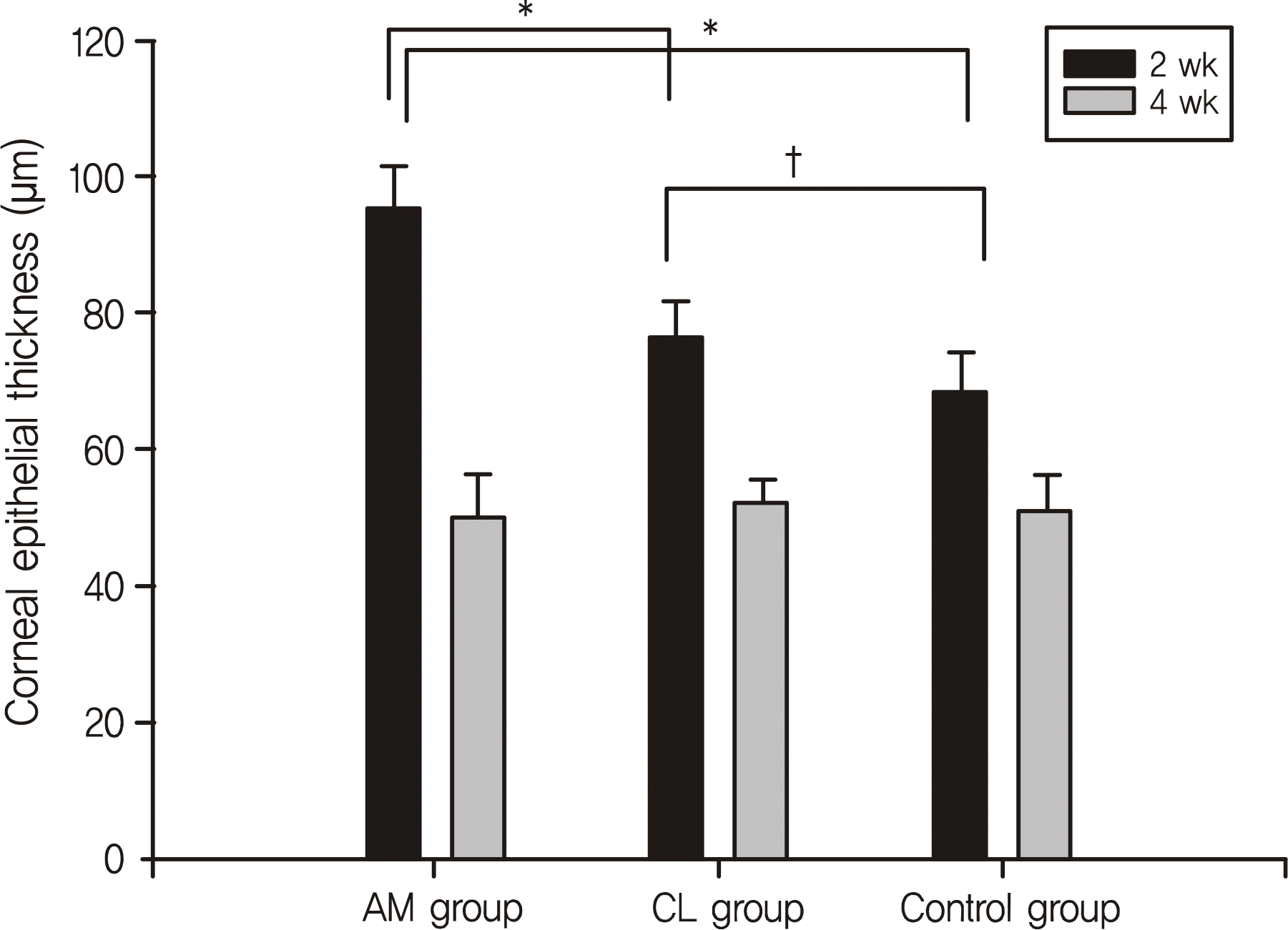 | Figure 5.Comparison of central corneal epithelial thickness among the 3 groups at 2 weeks and 4 weeks. In all groups, the central corneal epithelial thickness was significantly thicker at 2 weeks than at 4 weeks. * Corneal epithelial thickness in the AM group is significantly thicker than the CL and control group at 2 weeks. † Corneal epithelial thickness in the CL group is significantly thicker than the control group. AM = amniotic membrane; CL = contact lens. |
 | Figure 6.Transmission electron photomicrographs (original magnification ×45,000) of the central cornea at 2 weeks. (A) Morphologically established hemidesmosome is found in the amniotic membrane group. (B) Morphologically identifiable hemidesmosomes appear in the contact lens group. (C) Hyperactive formation of adhesion complex in the control group. |
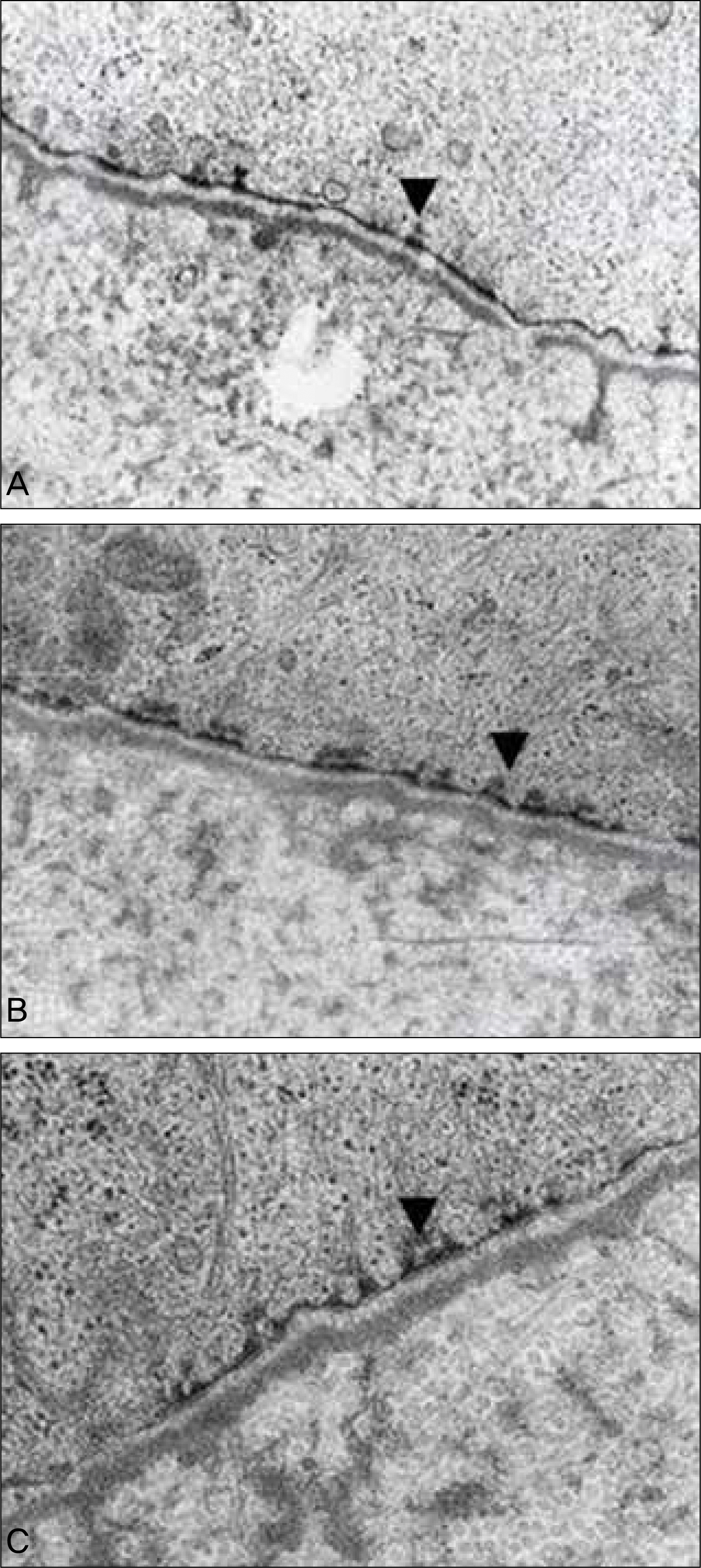 | Figure 7.Transmission electron photomicrographs (original magnification ×45,000) of the central cornea at 4 weeks. (A) Amniotic membrane group. (B) Contact lens group. (C) Control group. Morphologically established hemidesmosomes (arrowheads) are detected in all groups. There are no significant differences in adhesion complex formation among the 3 groups. |
Table 1.
The comparison of corneal epithelial thickness among the 3 groups 2 mm from the limbus (Corneal flap did not involve this area)
| Groups | n | Epithelial thickness (mean ± SD, μm) | p-value* |
|---|---|---|---|
| Control group | 9 | 51.4 ± 4.3 | |
| AM group† | 9 | 50.3 ± 3.3 | 0.73 |
| CL group‡ | 8 | 52.6 ± 3.3 |
Table 2.
The comparison of the central corneal epithelial thickness among the 3 groups
| Time | Control group (mean ± SD, n = 8) | AM group*(mean ± SD, n = 9) | CL group†(mean ± SD, n = 9) | p-value‡ |
|---|---|---|---|---|
| 2 weeks | 68.3 ± 6.1 | 95.3 ± 6.3 | 76.4 ± 5.1 | 0.012 |
| 4 weeks | 51.4 ± 4.7 | 50.0 ± 6.5 | 51.8 ± 3.9 | 0.612 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download