1. Chang MW, Kim SW, Oh IK, et al. Intravitreal triamcinolone injection with or without bevacizumab for diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1269–74.

2. Ahmadieh H, Ramezani A, Shoeibi N, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo-controlled, randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2008; 246:483–9.

3. Ehrlich R, Ciulla TA, Moss AM, Harris A. Combined treatment of intravitreal bevacizumab and intravitreal triamcinolone in patients with retinal vein occlusion: 6 months of follow-up. Graefes Arch Clin Exp Ophthalmol. 2010; 248:375–80.

4. Faghihi H, Roohipoor R, Mohammadi SF, et al. Intravitreal bevacizumab versus combined bevacizumab-triamcinolone versus macular laser photocoagulation in diabetic macular edema. Eur J Ophthalmol. 2008; 18:941–8.

5. Folgosa MS, Messias A, Takata C, et al. Single intravitreal injection of triamcinolone combined with bevacizumab for the treatment of diffuse diabetic macular oedema refractory to grid photocoagulation. Acta Ophthalmol. 2010; 88:e297–8.

6. Jonas JB, Hayler JK, Söfker A, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2001; 131:468–71.

7. Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003; 136:419–25.

8. Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001; 108:765–72.

9. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000; 20:244–50.

10. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.

11. Jonas JB, Kreissig I, Degenring R, et al. Secondary chronic open-angle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003; 121:729–30.

12. Roth DB, Realini T, Feuer WJ, et al. Short-term complications of intravitreal injection of triamcinolone acetonide. Retina. 2008; 28:66–70.

13. Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004; 138:740–3.

14. Jonas JB, Degenring RF, Kreissig I, et al. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005; 112:593–8.

15. Roth DB, Verma V, Realini T, et al. Long-term incidence and timing of intraocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmology. 2009; 116:455–60.

16. Jung JW, Nam DH, Shyn KH. The complications after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2007; 48:55–62.
17. Kim SJ, Park YM, Lee SU, et al. Short-term safety and efficacy of intravitreal bavacizumab injection. J Korean Ophthalmol Soc. 2009; 50:219–26.

18. Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin). Retina. 2007; 27:1044–7.

19. Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:779–81.

20. Shima C, Sakaguchi H, Gomi F, et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmologica. 2008; 86:372–6.

21. Ozkiriş A, Erkiliç K. Complications of intravitreal injection of triamcinolone acetonide. Can J Ophthalmol. 2005; 40:63–8.
22. Yang YH, Kim KR, Yang SW, Yim HB. The effect of intravitreal triamcinolone acetonide on intraocular pressure. J Korean Ophthalmol Soc. 2004; 45:1081–5.
23. Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004; 138:740–3.

24. Bakri SJ, Beer PM. The effect of intravitreal triamcinolone acetonide on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003; 34:386–90.

25. Park HY, Yi K, Kim HK. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Korean J Ophthalmol. 2005; 19:122–7.

26. Hattenbach LO, Klais C, Koch FH, Gümbel HO. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001; 108:1485–92.

27. Shin JH, Lee SW, Kim DK, et al. Intravitreal injection of triamcinolone acetonide for macular edema: long-term safety and efficacy after two years. J Korean Ophthalmol Soc. 2007; 48:1670–4.
28. Chung MS, Chang WH. Efficacy of anterior chamber paracentesis in intravitreal triamcinolone injection. J Korean Ophthalmol Soc. 2005; 46:1328–32.
29. Lee MW, Kyung SE, Chang MH. Prophylactic effect of brimoni-dine 0.15% on IOP elevation after intravitreal triamcinolone acetonide injection. J Korean Ophthalmol Soc. 2008; 49:743–52.

30. Seong HK, Lee JM, Park YS, Lee BR. Immediate natural course of IOP after IVTA and the effect of preoperative ocular massage. J Korean Ophthalmol Soc. 2007; 48:808–14.
31. Kahook MY, Kimura AE, Wong LJ, et al. Sustained elevation in intraocular pressure associated with intravitreal bevacizumab injections. Ophthalmic Surg Lasers Imaging. 2009; 40:293–5.

32. Jalil A, Fenerty C, Charles S. Intravitreal bevacizumab (Avastin) causing acute glaucoma: an unreported complication. Eye. 2007; 21:1541.

33. Kubota T, Okabe H, Hisatomi T, et al. Ultrastructure of the trabecular meshwork in secondary glaucoma eyes after intravitreal triamcinolone acetonide. J Glaucoma. 2006; 15:117–9.

34. Cho P, Lui T. Comparison of the performance of the Nidek NT-2000 noncontact tonometer with the Keeler Pulsair 2000 and the Goldmann applanation tonometer. Optom Vis Sci. 1997; 74:51–8.

35. Audren F, Lecleire-Collet A, Erginay A, et al. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: phase 2 trial comparing 4 mg vs 2 mg. Am J Ophthalmol. 2006; 142:794–9.

36. Lee JM, Kim SJ, Yi KY, Kim HK. Intraocular pressure change after secondary intravitreal triamcinolone acetonide injection. J Korean Ophthalmol Soc. 2007; 48:97–102.
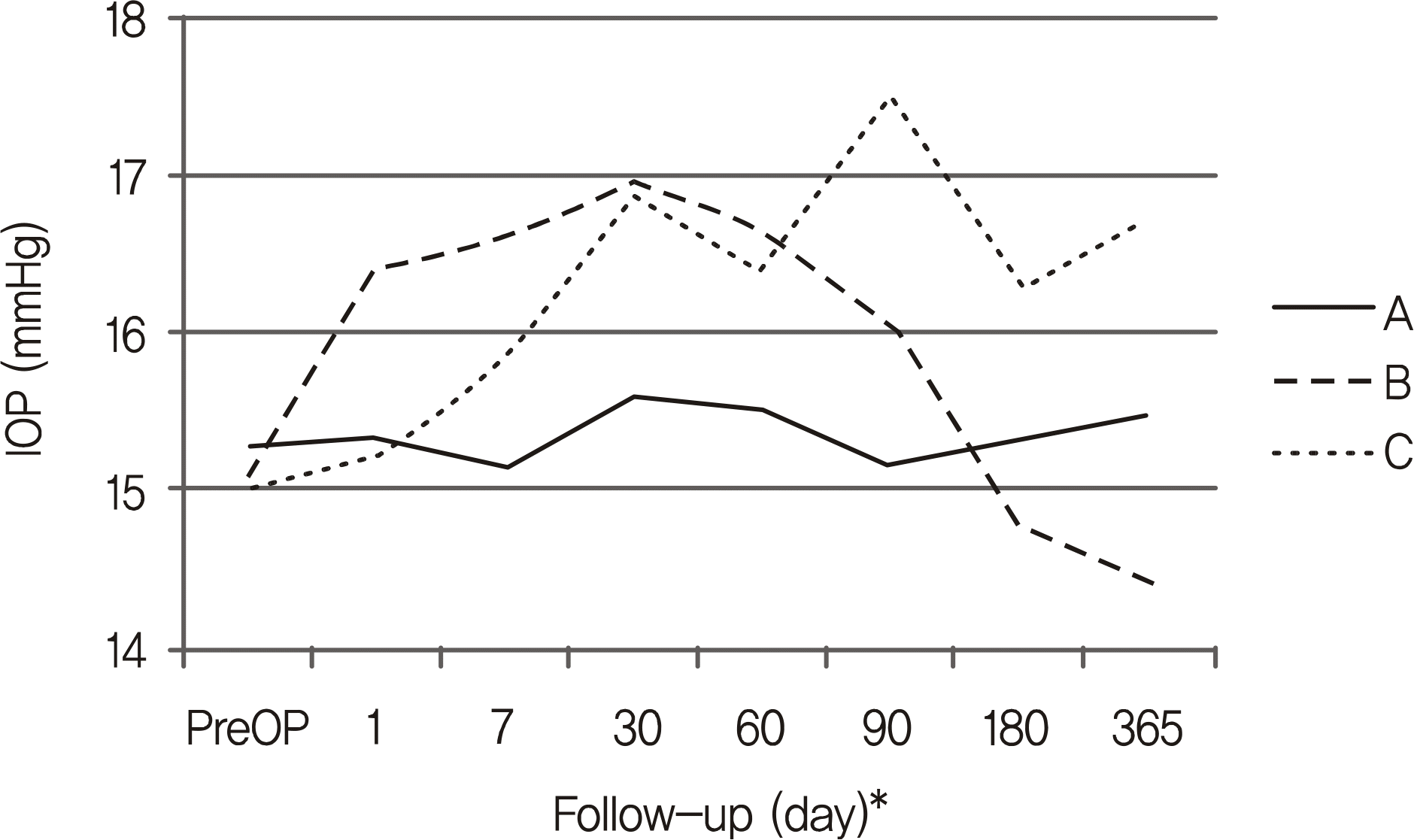
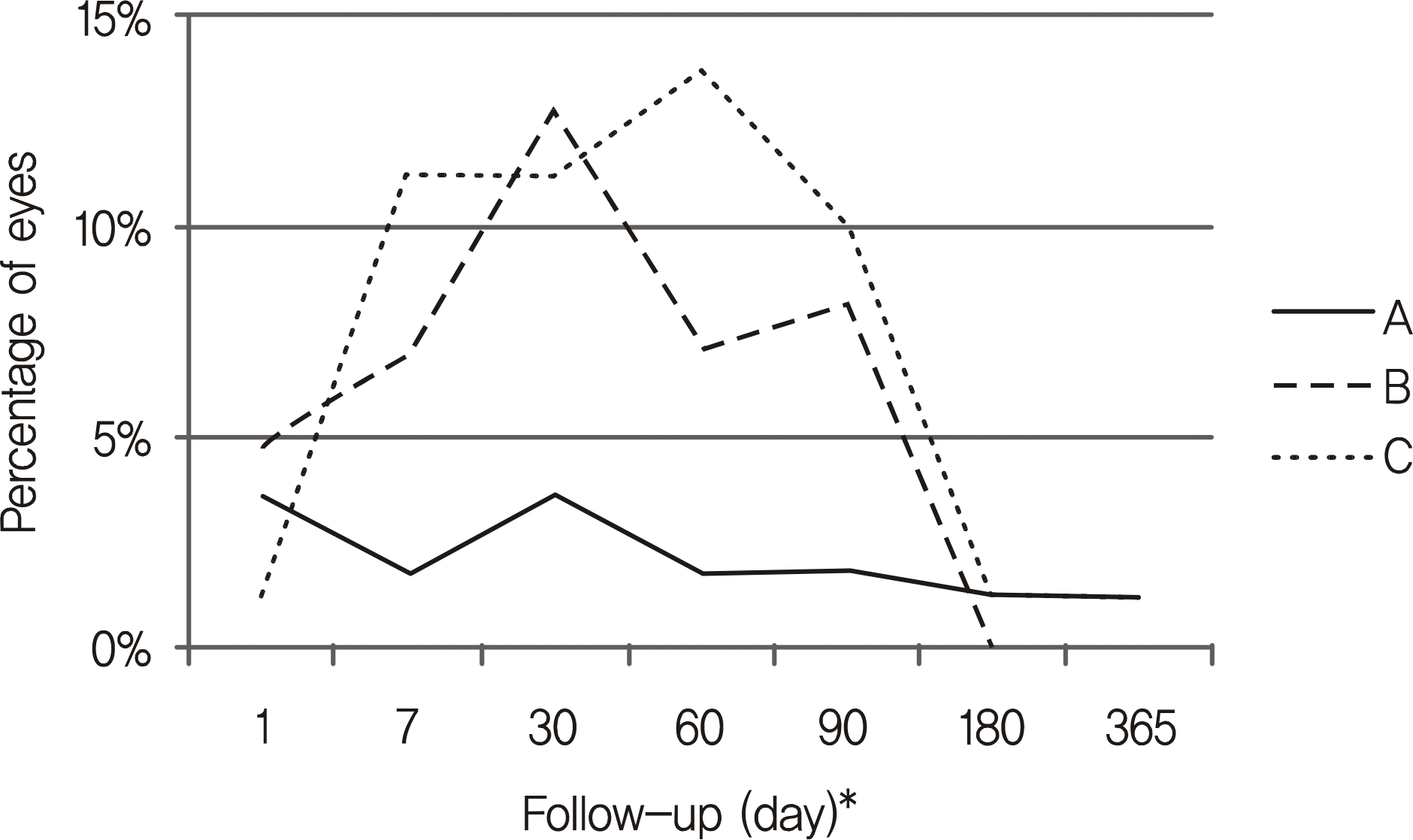




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download