Abstract
Purpose
To investigate the effects of 0.15% brimonidine tartrate ophthalmic solution spray on the luminal changes in the nasolacrimal excretory system.
Methods
A prospective study was performed on 52 eyes in 26 patients complaining of epiphora in both eyes. The ran-domly-assigned 26 test eyes (cases) received spray of the solution through the nasal cavity, and the other 26 eyes (controls) were irrigated with the same drug through the inferior calnaliculus. Dacryocystography was then performed to observe the luminal changes jn the nasolacrimal excretory system, patient symptoms and physiologic drainage functions.
Results
The changes in lumen width of the nasolacrimal duct (NLD) were noted, and the changes in lumen width of the lacrimal sac were not significant in either mode. The upper and middle parts of the NLD were widened more in the irrigation group, and the lower part of the NLD was widened more in the spray group. Though there was no significant difference in the physiologic drainage functions, the patients in both groups reported reduced symptoms.
Go to : 
References
1. Jones LT, Linn ML. The diagnosis of the cause of epiphora. Am J Ophthalmol. 1969; 67:751–4.
3. Mauriello JA Jr, Palydowycz S, DeLuca J. Clinicopathologic study of lacrimal sac and nasal mucosa in 44 patients with complete acquired nasolacrimal duct obstruction. Ophthal Plast Reconstr Surg. 1992; 8:13–21.

4. O'Donnell B, Shah R. Dacryocystorhinostomy for epiphora in the presence of a patent lacrimal system. Clin Experiment Ophthalmol. 2001; 29:27–9.
5. Lee TS, Kim JS, Cho SH, Choi JS. The surgical results of transcanalicular LASER-assisted dacryocystorhinostomy. J Korean Ophthalmol Soc. 2004; 45:1–7.
6. Kim YS, Lee TS. Clinical study conjunctivodacryocystorhinostomy with Jones tube. J Korean Ophthalmol Soc. 1991; 32:129–33.
7. Yun JR, Chang HK. Long-term follow-up of conjunctivodacry-ocystorhinostomy. J Korean Ophthalmol Soc. 1996; 37:1583–9.
9. Paulsen FP, Corfield AP, Hinz M, et al. Characterization of mucins in human lacrimal sac and nasolacrimal duct. Invest Ophthalmol Vis Sci. 2003; 44:1807–13.

10. Narioka J, Ohashi Y. Changes in lumen width of nasolacrimal drainage system after adrenergic and cholinergic stimulation. Am J Ophthalmol. 2006; 141:689–98.

11. Narioka J, Ohashi Y. Effects of adrenergic and cholinergic antagonists on diameter of nasolacrimal drainage system. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1843–50.

12. Kim YM, Oh DE. Effects in lumen width of nasolacrimal drainage system after adrenergic drug irrigation. J Korean Ophthalmol Soc. 2010; 51:1039–46.

13. Corboz MR, Rivelli MA, Varty LM, et al. Pharmacological characterization of postjunctional α-adrenoceptor in human nasal mucosa. Am J Rhinol. 2005; 19:495–502.
14. Paulsen F, Hallmann U, Paulsen J, Thale A. Innervation of the cavernous body of the human efferent tear ducts and function in tear outflow mechanism. J Anat. 2000; 197:177–87.

15. Paulsen FP, Thale AB, Hallmann UJ, et al. The cavernous body of the human efferent tear ducts: function in tear outflow mechanism. Invest Ophthalmol Vis Sci. 2000; 41:965–70.
16. Paulsen F, Thale A, Kohla G, et al. Functional anatomy of human lacrimal duct epithelium. Anat Embryol (Berl). 1998; 198:1–12.

17. Ayub M, Thale AB, Hedderich J, et al. The cavernous body of the human efferent tear ducts contributes to regulation of tear outflow. Invest Ophthalmol Vis Sci. 2003; 44:4900–7.

18. Lindstrὃ m AE. Contribution to the knowledge of the incidence and treatment of the diseases of the lacrymal passages. Acta Ophthalmol. 1923; 1:131–46.
20. Lang J. Innervation of the nasal cavity. In: Clinical Anatomy of the Nose, Nasal Cavity and Paranasal Sinus. 1st ed.New York: Thieme Medical Publishers;1989. p. 112–6.
21. Guzek JP, Ching AS, Hoang TA, et al. Clinical and radiologic lacrimal testing in patients with epiphora. Ophthalmology. 1997; 104:1875–81.

22. Massaro BM, Gonnering RS, Harris GJ. Endonasal laser dacryocystorhinostomy. A new approach to nasolacrimal duct obstruction. Arch Ophthalmol. 1990; 108:1172–6.
Go to : 
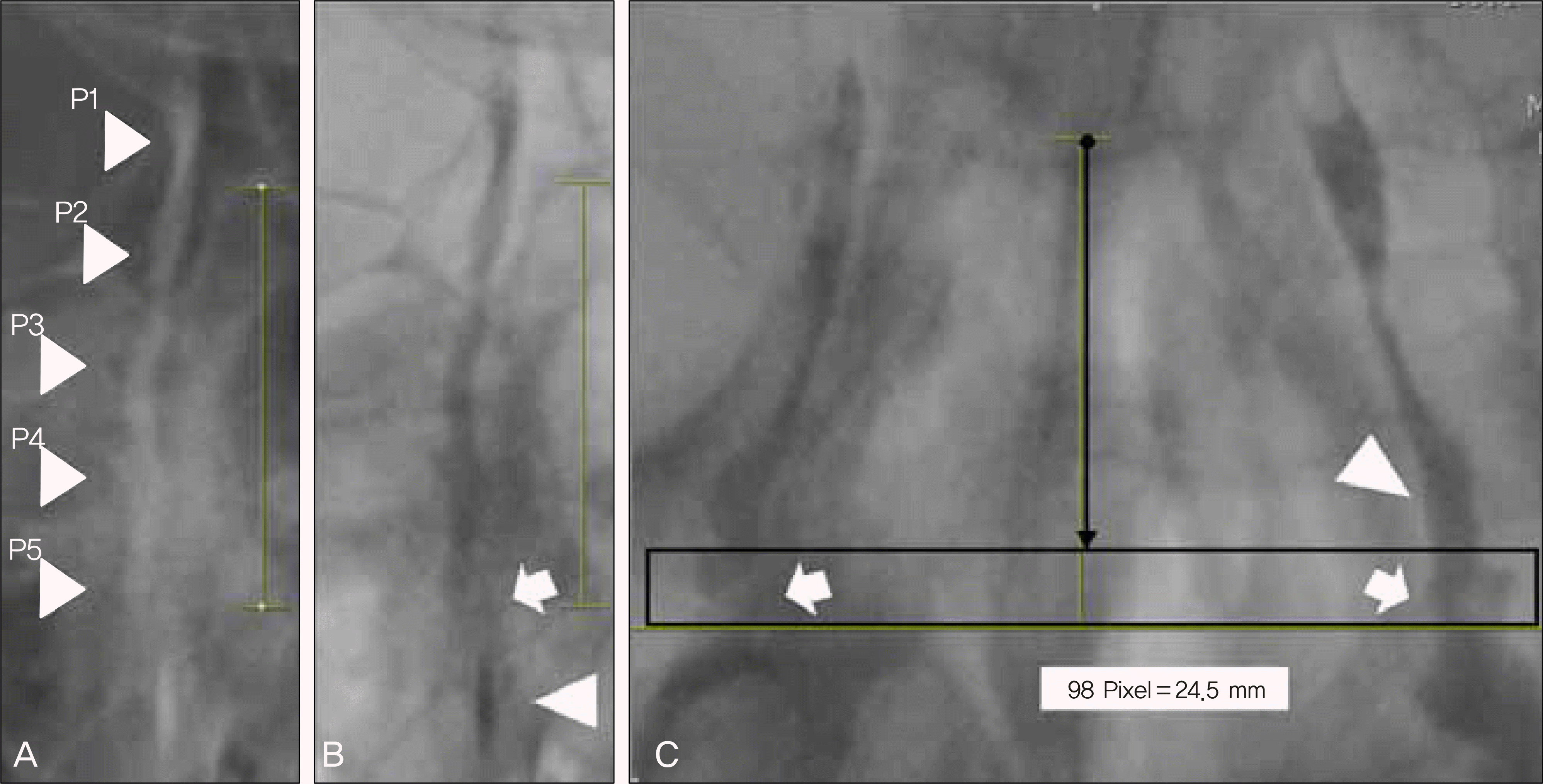 | Figure 1.(A) The five points at the positive image of dacryocystography. (B) Intense inhancement of intraductal dye contrast (arrowhead) and disconnected area of intraductal flow (arrow) at the negative image of dacryocystography. (C) The laminal flow (arrowhead) is changing into the turbulent flow with multiple air bubbles (arrows) at the transitional zone (box). The distance (about 24.5 mm) between common canaliculus and the fifth point is similar on both sides. |
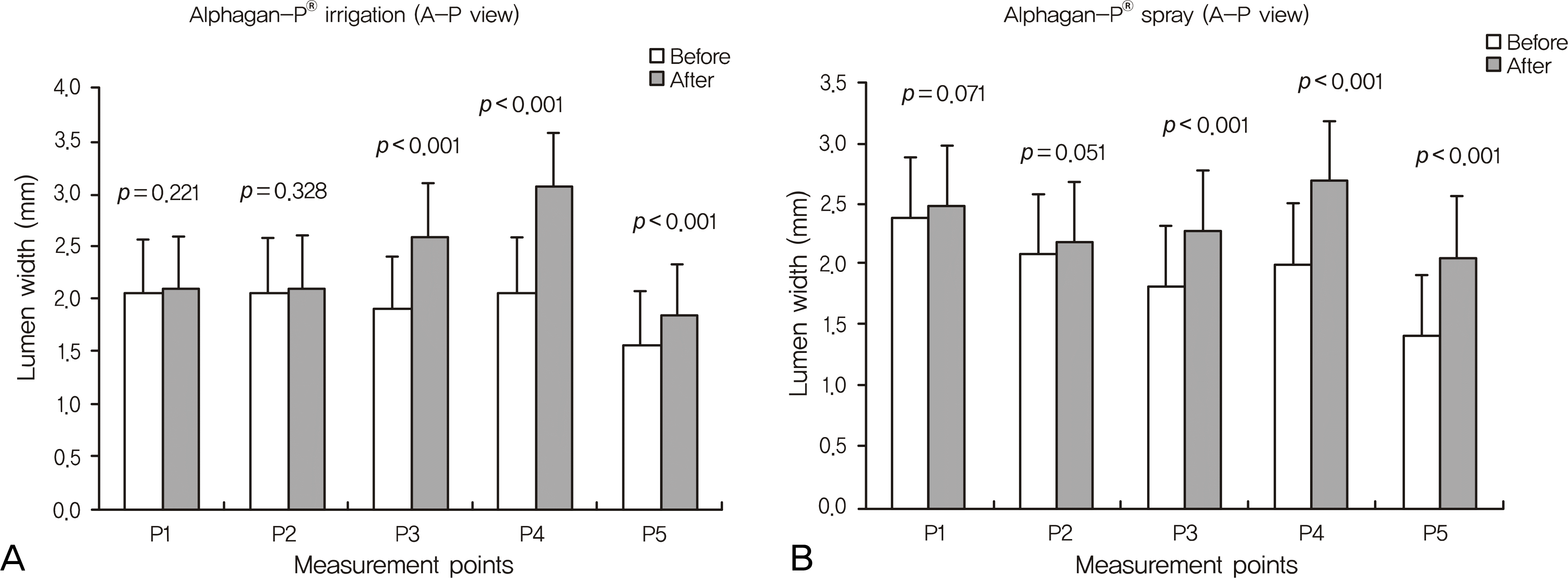 | Figure 2.Each graph shows luminal changes (mm) in the lacrimal excretory system at 5 points in Alphagan-P® irrigation group and spray group at anteroposterior images. |
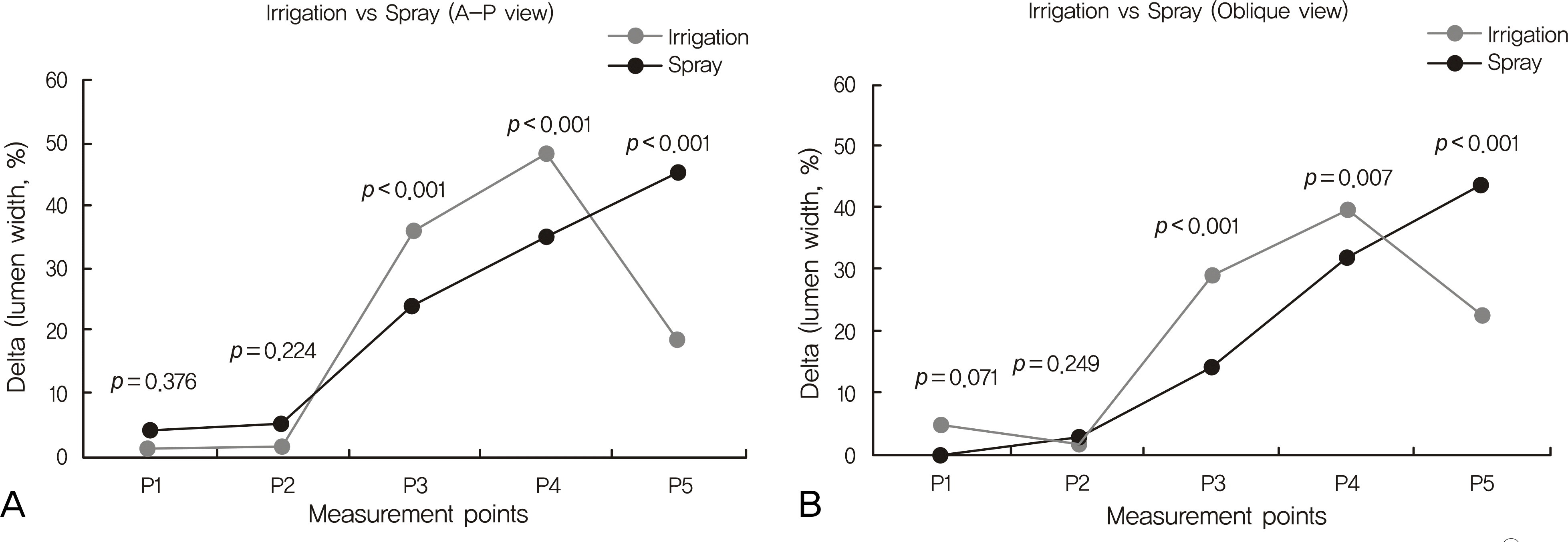 | Figure 3.Each graph shows the comparison of the increasing rate (%) of the lumen width at 5 points between in Alphagan-P® irrigation group and spray group at anteroposterior and oblique images. (One way ANOVA test for statistical analysis) |
Table 1.
The characteristics of patients
| Irrigation (mean ± SD, n = 24) | Spray (mean ± SD, n = 24) | p-value* | |
|---|---|---|---|
| Age (yr, average) | 63.5 ± 7.5 | ||
| Sex (M/F) | 22/2 | ||
| Intensity of symptoms (point, 1-5) | 2.9 ± 1.8 | 2.8 ± 1.6 | 0.424 |
| Duration of symptoms (point, 1-5) | 3.2 ± 1.0 | 3.3 ± 1.3 | 0.091 |
| Schirmer I test | 13.7 ± 3.0 | 14.1 ± 4.0 | 0.062 |
| Dye disappear test | 2.0 ± 0.3 | 2.1 ± 0.3 | 0.103 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download